THE enthalpy is a thermodynamic function by which calculates the heat involved in isobaric processes, that is, that are kept under constant pressure. Its development took place shortly after the fall of the caloric theory, with the advancement of thermodynamics between the years 1840 and 1850.
The enthalpy, for the whatumica, does not have much meaning if it is worked as an absolute and isolated value, but it does when considering the variation of its value in a chemical process. There are several ways to calculate the enthalpy variation of a process, the main ones being through the energies of formation, binding and also by the therehey from hess.
Read too: What is Gibbs Free Energy?
Enthalpy Summary
Enthalpy is a thermodynamic tool for calculating the heat involved in processes that occur at constant pressure.
It was defined by American physicist Josiah W. Gibbs, in the context of the fall of the caloric theory.
In chemistry, we always use the enthalpy change, represented as ΔH.
Chemical reactions that absorb heat are called endothermic and have ΔH > 0.
Chemical reactions that release heat are called exothermic and have ΔH < 0.
Video lesson on enthalpy
What is enthalpy?
The enthalpy, always represented by H, was initially defined by American physicist Josiah Willard Gibbs, which he called the heat at constant pressure function, since, in his words:
“[…] the decrease in function represents, in all cases where the pressure does not vary, the heat given up by the system.”
Do not stop now... There's more after the advertising ;)
From Gibbs' work, then, we can understand enthalpy as a thermodynamic function whose variation is numerically equal to the amount of heat exchanged in the system, to pressure constant. This means that, in isobaric processes (like most chemical processes), knowing calculate the variation of the enthalpy function, one can then know the value of heat exchanged between system and neighborhood.

Such a correlation with heat causes many students mistakenly think that enthalpy is synonymous with heat or something like energy content, heat content, released heat and absorbed heat, and the like.
The enthalpy arose in the context of the collapse of the caloric theory, which treated heat as an imponderable material substance that was transferred from warmer bodies to colder bodies. Thus, there was a need to have a new tool for calculating heat. The solution, then, was to use quantities that already had defined thermodynamic equations, such as enthalpy.
Read too: How to calculate the enthalpy change of solution?
enthalpy variation
Since enthalpy is a tool used to calculate the heat exchanged in a chemical process, it makes no sense to use it as an absolute, isolated number, but considering its variation, that is, in practice, we should only assess how much, numerically, the enthalpy varied during the chemical process, since thermodynamics assures us that its variation is numerically equal to the heat released or absorbed in the process.
Strictly speaking, we can define the enthalpy variation as:
ΔH = HFinal - Hinitial
As in chemical processes, the final step can be considered the products and the initial step can be considered the reagents. It is also common to see the definition of enthalpy variation as:
ΔH = Hproducts - Hreagents
From a practical and interpretive point of view, if the enthalpy change is positive (ΔH> 0), we say the chemical reaction is endothermic, that is, there is heat absorption throughout the process. Already if the enthalpy change is negative (ΔH< 0), we say the chemical reaction is exothermic, that is, heat is released throughout the process.
The enthalpy variation, in many cases, is observed in graphs, as shown in the following examples.
Example 1:
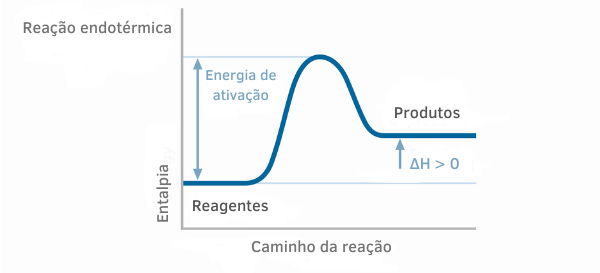
Endothermic Reaction Chart
In the enthalpy graphs for endothermic reactions, it can be seen that the amount of enthalpy of the products is greater than that of the reactants, indicating that the variation along the reaction is positive. Thus, if ΔH > 0, we can say that the chemical process occurred with heat absorption.
Example 2:
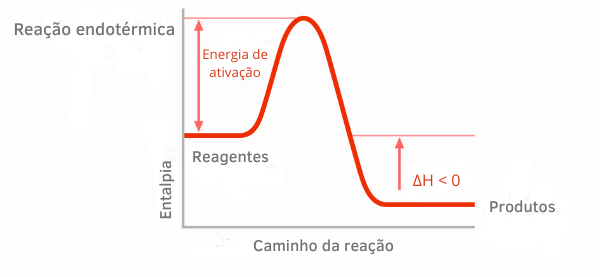
Exothermic Reaction Chart:
In the enthalpy graphs for exothermic reactions, it can be seen that the amount of enthalpy of the products is smaller than that of the reactants, indicating that the variation along the reaction is negative. Thus, being ΔH < 0, we can say that the chemical process occurred with the release of heat.
Read more about these classifications of chemical reactions in the text: FORendothermic and exothermic processes.
Types of enthalpy
formation enthalpy
THE formation enthalpy é calculated based on the formation chemical reactions, which are reactions in which one mole of compound substances is formed from their most stable simple substances at room temperature and 1 atmosphere of pressure.
H2(g) + ½ O2 (g) → H2O (l) H°f = -286 kJ/mol
The great advantage of the enthalpy of formation is that simple substances that are more stable at room temperature and 1 atmosphere of pressure have an enthalpy agreed to zero. This is not to say that they are actually zero, but, for simplification and better classification, they are treated like this.
Being H = Hproducts - Hreagents, if we consider, then, that Hreagents = 0, we can say that the observed value of ΔH is only related to the products, which, in these cases, are always one mole of the compound substance. Therefore, we table this value as the standard enthalpy variation of water formation, represented by ΔH°f.
It was with this methodology that several substances had their variations of standard enthalpy formation tables, as we can see below.
Substance |
Formation enthalpy (ΔH°f) in kJ/mol |
CO2 (g) |
-393,4 |
CaO(s) |
-634,9 |
HI(g) |
+25,9 |
NO (g) |
+90,1 |
binding enthalpy
The binding enthalpy serves to indicate the amount of energy involved in the rupture or formation of a given mole. chemical bond.
It is understood that, to break a chemical bond, it is necessary to absorb heat, so that the bonding atoms increase their internal energy and, consequently, increase your kinetic energy. with bigger kinetic energy, the atoms vibrate more intensely, causing the bonds to break. Thus, every bond breaking is an endothermic process.
Otherwise, to form a chemical bond, atoms lose freedom of movement and need to decrease their degree of movement, decreasing their kinetic energy. The spare energy is then released in the form of heat. Thus, all bond formation is an exothermic process.
The table below shows values of the energies associated with each chemical bond.
Connection |
Binding energy (kJ/mol) |
C-H |
412,9 |
C-C |
347,8 |
O═O |
497,8 |
F-F |
154,6 |
NO |
943,8 |
Note that there are no signs in the values, as they are in modulus. This is because the signal must be assigned by you depending on whether the link is broken or formed.
Combustion enthalpy
THE combustion enthalpy serves to indicate the amount of heat released in the combustion of one mole of a substance. It should be noted that every combustion reaction is exothermic, as every burn releases heat.
CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2The ΔH°Ç = -889.5 kJ/mol
The table below shows enthalpy of combustion values for some chemical substances.
Substance |
Combustion enthalpy (ΔH°Ç) in kJ/mol |
Ethanol - C2H5OH (1) |
-1368 |
Benzene - C6H6 (1) |
-3268 |
Octane - C8H18 (1) |
-5471 |
Glucose - C6H12O6 (s) |
-2808 |
Enthalpy of physical state change
Every change in physical state involves heat exchange. Therefore, the enthalpy of change of physical state serves to indicate the amount of heat involved in physical state change processes.
For example, we have water vaporization:
H2O (1) → H2O (g) ΔH = +44 kJ/mol
In the melting of water, we have:
H2O(s) → H2O (l) ΔH = +7.3 kJ/mol
Enthalpy values are symmetric for inverse processes, which means that, for example, the enthalpy change in the liquefaction of water is -44 kJ/mol, while, in its solidification, it is equal to -7.3 kJ/mol.
Read too: What is entropy?
Solved exercises on enthalpy
Question 1 - (UERJ 2018) The polluting capacity of a hydrocarbon used as fuel is determined by the ratio between the energy released and the amount of CO2 formed in its complete combustion. The higher the ratio, the lower the polluting capacity. The table below shows the standard enthalpy of combustion of four hydrocarbons.
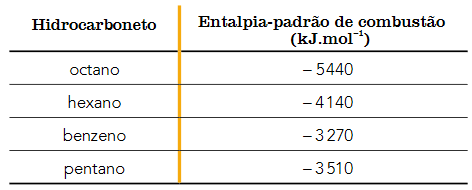
From the table, the hydrocarbon with the lowest polluting capacity is:
Octane
Hexane
Benzene
pentane
Resolution
Alternative D
The question indicates that the polluting capacity is defined as the ratio (quotient) between the energy released and the amount of CO2 formed in its complete combustion. the larger the reason, the lower the polluting capacity, that is, more energy is released per mole of CO2 generated.
The complete combustion reactions of Hydrocarbons cited are:
Octane: Ç8H18 +25/2 O2 → 8 CO2 + 9 hours2The Reason: 5440/8 = 680
Hexane: Ç6H14 +19/2 O2 → 6 CO2 + 7 hours2The Reason: 4140/6 = 690
Benzene: Ç6H6 + 15/2 O2 → 6 CO2 + 3 H2The Reason: 3270/6 = 545
pentane: Ç5H12 + 8 O2 → 5 CO2 + 6 H2The Reason: 3510/5 = 702
Thus, we can conclude that pentane is the hydrocarbon with the lowest polluting capacity.
Question 2 - (Enem 2015) The use of forest residues is becoming more attractive every day, as they are a renewable source of energy. The figure represents the burning of a bio-oil extracted from wood waste, where ΔH1 the enthalpy variation due to the burning of 1 g of this bio-oil, resulting in carbon dioxide and liquid water, and ΔH2 the enthalpy change involved in the conversion of 1 g of water in a gaseous state to a liquid state.

The enthalpy variation, in kJ, for burning 5 g of this bio-oil, resulting in CO2 (gaseous) and H2The (gaseous) is:
A) -106
B) -94
C) -82
D) -21.2
E) -16.4
Resolution
Alternative C
From the graph shown, we have ΔH1 as the enthalpy variation of bio-oil burning producing CO2 (g) and H2O (1) and ΔH2 as the enthalpy change of water liquefaction, since the CO2 remains gaseous and only the physical state of the Water changes (from gas to liquid).
The exercise asks for the enthalpy change of burning 5 g of bio-oil, resulting in CO2 (gaseous) and H2O (gaseous). From the diagram, this enthalpy change can be defined as ΔH = ΔH1 – H2. Thus, the value of ΔH will be equal to -16.4 kJ/g. This variation, as we can see in the unit, is for EACH gram of bio-oil. For 5 grams, we must do the proportion:
1 g of bio-oil -16.4 kJ
5 g of bio-oil x
1. x = 5. (-16,4)
x = -82 kJ
We can then mark alternative C.
By Stéfano Araújo Novais
Chemistry teacher