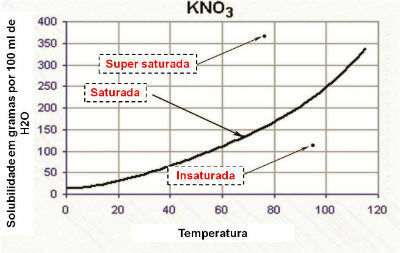
THE concentration in quantity of matter is the relationship between the amount of matter in the solute, measured in mol (n1), and the volume of solution in liters (V). This concentration is measured in mol per liter (mol/L).
Let us consider the gastric juice that our stomach produces for the purpose of carrying out the digestion process. In reality, it is a solution of hydrochloric acid (HCl) at a concentration of 0.01 mol/L. This means that for every liter of gastric juice there is 0.01 mol of HCl.
THE concentration in quantity of matter it is often called by some authors molar concentration or molarity, but the correct terms are “concentration in mol/L” or “concentration in quantity of matter”. Furthermore, this concentration is the most recommended by the International System of Units (SI) and by the International Union of Pure and Applied Chemistry (IUPAC); therefore, it is the most used in laboratories and chemical industries.
Mind Map: Concentration Unit in Mol/L

* To download the mind map in PDF, Click here!
Formulas used in molarity
The mathematical formula used to calculate this concentration is given by:

In many cases, the value of the amount of matter in the solute is not given, but its mass expressed in grams (m1). In these cases, we have that the amount of matter in the solute in moles (n1) can be achieved by dividing the mass of the solute by the molar mass of the solute itself, according to the following formula:
Do not stop now... There's more after the advertising ;)

Replacing n1 in the equation, we have:

Example of molarity calculation
Consider the following example to see how this calculation is done:
“A 100 ml aqueous solution contains 20 g of NaCl. How to proceed to express the concentration of this solution in amount of matter per volume?”
Resolution:
Well, the formula to be used is the same as shown above, but the volume is not in liters. So, we must do the following unit conversion:
1 L 1000 ml
V 100 mL
V = 0.1 L
It is also necessary to find the molar mass value of the NaCl salt. To do this, it is necessary to know the values of the atomic masses of both elements and perform the molar mass calculation, which is taught in the text “Molar Mass and Mol Number”:
M (NaCl) = 1. 23 + 1. 35, 46
M (NaCl) = 58.46 g/mol
Now we can replace all values in the formula and find the concentration value in mol/L:
m = no1
M1.V
M = 20
58,46.0,1
M = 3.4 mo/L
*Mental Map by Me. Diogo Lopes
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Concentration in mol/L or molarity"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/concentracao-mol-l-ou-molaridade.htm. Accessed on June 27, 2021.