Every cell is a device in which a spontaneous oxidation-reduction reaction occurs that generates an electrical current, which, in turn, is used to make some equipment work.
These devices got their name because the first battery to be created was invented by Alessandro Back in the year 1800, it was formed by zinc and copper discs separated by cotton soaked in brine. This set was placed interspersed, one on top of the other, piling up the discs and forming a large column. How was a battery of discs, began to be called by that name.

The batteries are always made up of two electrodes and an electrolyte. The positive electrode is called a cathode and this is where the reaction of reduction. The negative electrode is the anode and this is where the reaction of oxidation. Electrolyte is also called salt bridge and is the ion-conducting solution.
For you to understand how this generates electrical current, see the case of one of the first batteries, the Daniell's pile, in which there was a container with a solution of copper sulfate (CuSO
4(aq)) and, dipped in that solution, was a copper plate. In another separate container, there was a solution of zinc sulfate (ZnSO4(aq)) and a dipped zinc plate. The two solutions were connected by a salt bridge, which was a glass tube with a potassium sulfate solution (K2ONLY4(aq)) with glass wool at the ends. Finally, the two plates were interconnected by an external circuit, with a lamp, whose lighting would indicate the passage of electrical current: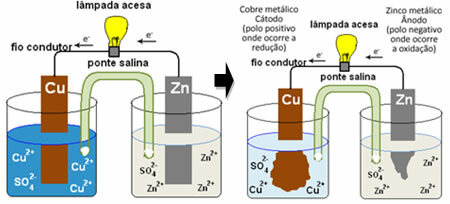
What happens is that zinc has a greater tendency to oxidize, that is, to lose electrons, so the metallic zinc of the blade works as the negative electrode, the anode, where oxidation occurs: Zn( s) ↔ Zn2+(here) + 2 and-. The electrons lost by the zinc are transported by the external circuit to the copper, generating the electric current that turns the lamp on. The copper ions in the solution receive electrons (reduce) and transform into metallic copper that is deposited on the copper sheet. This means that this is the positive electrode, cathode, where the reduction occurs: Ass2+(here) + 2 and- ↔ ass( s).
Today's batteries have this same operating principle, in which one metal donates electrons to another, through a conductive solution, and an electric current is produced. The difference is that the batteries used today are dry, because they do not use a liquid solution as electrolyte, as occurs in Daniell's battery.
Today there is a very wide variety of batteries that are sold commercially. Among them the most common are the acid cells (from Leclanché) and thealcaline batteries.
Both have zinc as the negative electrode; on the other hand, as a positive pole, there is a graphite bar installed in the middle of the pile surrounded by manganese dioxide (MnO2), powdered charcoal (C) and a wet paste. The difference is that, in the acid pile, ammonium chloride (NH) is used in the wet paste.4Cl) and zinc chloride (ZnCl2) - salts of acidic character - in addition to water (H2O). In the alkaline battery, potassium hydroxide (KOH), which is a base, is used.

Leclanché batteries are best suited for equipment that requires light and continuous discharge, such as remote control, wall clock, portable radio and toys. Alkaline batteries, on the other hand, have 50 to 100% more energy than a common battery of the same size, being recommended for equipment that require faster and more intense downloads such as radios, CD/DVD players, portable MP3 players, flashlights, digital still cameras etc.

Learn more about what electrolytes and electrodes are used in these and other types of batteries as well. as for what types of equipment they are most recommended, reading the related articles more bellow.
By Jennifer Fogaça
Graduated in Chemistry
