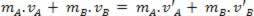Today we know that heat it means transferring energy from one object or system to another, due to the difference in temperature between them. But this concept has not always prevailed. Before him, many other concepts were elaborated, but all were discarded.
In the 18th century, the concept of heat placed it as a substance and not as energy. Initially, it was considered a kind of invisible substance or fluid. Regarding this substance, it was said that the greater the amount of heat in an object, the higher the temperature of that object. If the object was isolated, it was said to hold this substance, heat, keeping its temperature the same.
When two objects of different temperatures were in contact, it was believed that there would be a fluid exchange, and the fluid it went from the hottest body to the coldest body, until their temperatures were equal, that is, until equilibrium was reached thermal. When temperatures evened out, the process stopped. This theory also considered that heat was attracted to matter and its total amount was constant: it could neither be created nor destroyed.
Some processes were well explained by theory of heat as substance, other phenomena were not correctly explained, as it was necessary to admit that this substance (heat), also called caloric, it had very special characteristics: it penetrated easily into matter, was attracted to it, could neither be created nor destroyed and could not it had mass.
Physics seeks to satisfactorily explain the greatest possible amount of physical phenomena around us. Thus, the question remained: how could the caloric theory explain the heating caused by friction between two objects?
When we rub our hands constantly, we notice that they get warm. We also notice this heating when we drill a metal drill. Therefore, we can say that this heating is related to friction between two objects. Thompson, in the 18th century, realized that, when drilling a hole in the barrel of a metal cannon, high heating was produced. This heating was nothing more than the amount of caloric being increased.
The hypothesis that all that heat was already in the piece would lead to the conclusion that the cannon should melt even before being punctured, which was absurd. It was Thompson who reworked the concept of heat as the movement of particles that make up metals. Despite this, the theory of heat as a substance was accepted by scientists throughout the 18th century and, on a daily basis, we often consider heat as a substance.
Do not stop now... There's more after the advertising ;)
By Domitiano Marques
Graduated in Physics
Would you like to reference this text in a school or academic work? Look:
SILVA, Domitiano Correa Marques da. "Heat as a substance"; Brazil School. Available in: https://brasilescola.uol.com.br/fisica/calor-como-substancia.htm. Accessed on June 27, 2021.