Carbon Chain is defined as the set of all carbon atoms, as well as heteroatoms that make up the molecule of any organic compound. They can be classified into:
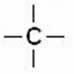
The) saturated chain: when it has only single bonds between carbon atoms. These bonds are called sigma (σ).

We say that the carbon present in this chain is saturated, as it has four single bonds.
Do not stop now... There's more after the advertising ;)
B) unsaturated chain: when it has at least one double bond (=) or one triple bond (≡). The bonds present are known as pi (π) bonds.
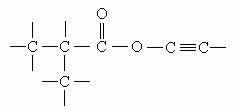
The triple bond at the right end of the chain characterizes it as unsaturated. The oxygen inserted between the two carbon atoms is classified as a heteroatom.
By Líria Alves
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Classification of carbon chains: types of bond"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/classificacao-das-cadeias-carbonicas-tipos-ligacao.htm. Accessed on June 28, 2021.


