There are three types of alkadienes or dienes, which are:
1-Accumulated: The double bonds are located on the same carbon and appear successively:
─C ═ Ç ═ C ─
│ │
2- Isolated: The double bonds are on different carbons and are separated by at least two successive single bonds:
│
─C ═ C C ─ C ═ C ─
│ │ │ │ │
3- Conjugates: Double bonds appear alternately, being separated by a single single bond:
─C ═ C C ═ C ─
│ │ │ │
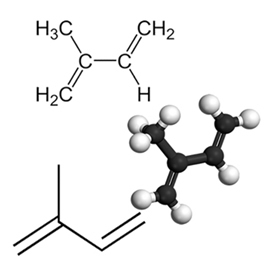
An example of an important conjugated diene is isoprene, which is a basic unit of the terpene group, a class of organic compounds. very important, some examples of products formed by isoprenes are rubber, beta-carotene (responsible for the orange color of the carrots), vitamin A and oils found in fruit peels, seeds, flowers, leaves, roots, vegetables and wood, such as limonene and myrcene.
To the accumulated and isolated dienes, addition reactions occur just like in the case of alkenes, what you can understand by reading the text Addition Reactions. The only difference is that dienes undergo this type of double reaction, because they have two double bonds, while alkenes have only one double bond.
However, in the case of conjugated dienes, the addition reaction has a particularity, as these compounds can undergo resonance, so the addition can take place in two ways:
1- Addition 1.2:
This is considered normal addition, as the addition occurs on the two carbon atoms that are making the same double bond, that is, on carbon 1 and 2:
Do not stop now... There's more after the advertising ;)
H2Ç ═ CH─ CH ═ CH2 + HBr → H2C CH─ CH ═ CH2
│ │
HBr
See that this type of reaction follows the Markovnikov's rule, the hydrogen bonds to the more hydrogenated carbon (with more hydrogen atoms attached).
Addition 1,2 is processed at low temperatures (-60°C).
2- Addition 1.4:
In this case, the reaction proceeds at high temperatures. It is important to point out that both the product of addition 1.2 and addition of 1.4 are always formed, but the temperature indicates which will be formed in greater quantity.
The following is an example of addition of type 1.4:
H2Ç ═ CH─ CH ═ CH2 + HBr → H2C CH ═ CH─CH2
│ │
HBr
Note that the following occurs, one of the shared electrons in the pi bond of each of the double carbons are shared with the substituent and the others form a new double bond between other carbons:
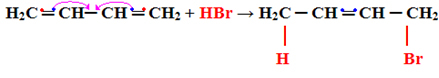
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Addition Reactions in Dienes"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/reacoes-adicao-dienos.htm. Accessed on June 28, 2021.