O washing powder it is a cleaning product that is part of the daily life of a large portion of the population, as it is used to facilitate the washing of people's clothes. It is a product that replaced bar soap, since, in the washing process in general, people had the habit of rubbing it on their clothes.

Bar soap is still widely used in dish washing, for example
Powder soap was first manufactured in 1946, after some attempts to make the use of bar soap easier. One of the attempts was to manufacture the soap in flakes or granules. However, these attempts were not successful because the soap interacts with ions present in hard water, mainly impairing the cleaning action.
Why can't we call laundry detergent that way?
Chemically speaking, we cannot call laundry detergent that way. If we look at the packaging of these products, we will see that it says washing machines, not washing powder. It is correct to call it Powder detergent, as its chemical composition is different from the composition of a soap.
The chemistry of washing powder, or better, washing powder, is based on the basic difference between a soap and a detergent. See the definition and chemical characteristics of these materials:
a) Detergents:
They are chemical substances that have very long chains (large amount of carbons) constituted only by carbon and hydrogen atoms, configuring what we call regions non-polar. Also, at the end of this long chain, there is a polar group.
The polar part of the detergent is due to the presence of a sulfonate group, that is, a sulfur atom interacting with three oxygen atoms, or a phosphate group, which has a phosphorus atom interacting with three atoms of oxygen. See the representation of the structure of a detergent:
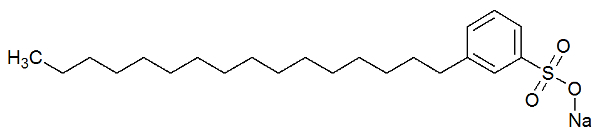
Structural formula of a detergent
The polar part (formed by sodium sulphonate) of the detergent is capable of interacting with water molecules. The non-polar part (formed only by carbons and hydrogens) interacts with the fat molecules. Thus, the detergent makes the fat interact with the water and form an emulsion.
Do not stop now... There's more after the advertising ;)
b) Soaps
are salts of carboxylic acids originated from the chemical reaction between fats and strong bases, such as NaOH. See the representation of the chemical structure of the soap:
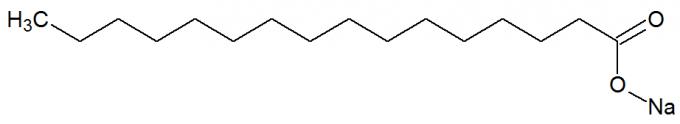
Structural formula of a soap
They also have long carbon chains with a nonpolar and polar part. In this way, the non-polar part (made up only of carbons and hydrogens) of the soap is able to interact with fats, and its polar part (formed by the COONa group), with water, forms emulsions, such as detergents.
As the focus of this text is the chemistry of “soap” powder, or rather, the chemical composition of the powder detergent, we present all the chemical substances that are part of its composition, as well as the importance of each one of them:
Chemical Composition of Powder Detergent
anionic surfactant (such as sodium alkyl benzene sulfonate and sodium alkyl ether sulfonate). They bind to the fat molecule as well as the water molecule, thus removing the fat from the tissue;
Enzymes: Lipases and proteases are used to help remove stains. This is because, chemically, enzymes are biochemical catalysts that promote the transformation of complex molecules into simpler molecules. Thus, smaller molecules can be more easily removed from clothing;
Bleach (Sodium Perborate): It acts by oxidation, reduction or enzymatic action. In water, it produces hydrogen peroxide, which is a powerful oxidizing agent. They chemically react with the garment's pigment, modifying the structure and resulting in a color change;
Optical Blockers: they are substances that absorb ultraviolet radiation or ultraviolet light and, shortly thereafter, emit a blue fluorescent light, masking, for example, the yellowish color of clothing;
Fragrances: They are essences used to leave a pleasant odor on clothes after the washing process. It is noteworthy that fragrances are essences (they belong to the ester function);
Dyes: Substances used to color the product;
Sequestering and chelating agents: EDTA (ethylenediamine tetraacetic acid) is an example of a sequestrant. They interact with calcium, magnesium and iron ions present mainly in hard water, not allowing the interaction of any cleaning action component, such as the surfactant.
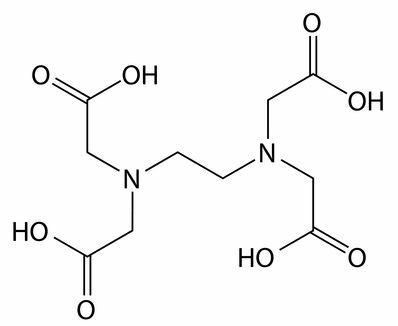
Structural formula of the EDTA sequestrant
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Chemistry of Powder Soap"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/quimica-sabao-po.htm. Accessed on June 28, 2021.