When drinking cold water, our body burns calories, bringing it to thermal equilibrium with the body at a temperature of about37°C. Thus, it is natural to imagine that the intake of Watercoldhelp with weight loss, but this concept is wrong.
All the foods we eat provide us with the energy needed for the body's vital functions. Although there are exceptions, the human body needs, average, from an intake of 2000kcal (kilocalories) daily. In practice, this means that if our body consumes morecalories than we ingest, we lose weight.
In this context, what is the impact of a glass of ice water on our daily calorie intake? For this, we will use a well-known equation: a fundamental equation gives calorimetry, used to calculate the sensible heat.
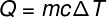
In the equation above, Q is the amount of heat, which can be measured in calories (lime) or joules (J); m and the pasta of the body in grams (g); ç it's the specific heat of the water in cal/g °C (or cal/g. K); and ΔT and the variationintemperature suffered by the body in °C or K (the temperature variation in these two measurements is equal).
Based on the equation, let's answer the following question: How many calories does it take to heat a glass of ice water to 4°C, in 200 ml, until the equilibrium temperature with the human body (37°C)? The specific heat of water is 1.0 cal/g °C, which means that to vary the temperature of 1 g of water in 1°C, consumes itself 1 cal. Note the calculation below:

In the calculation above, we used 200 g for the mass of water, as its density is 1.0 g/ml. The result indicates that if we ingest 200 ml from water to 4°C, will be consumed at least 6,8kcal (6800 cal). In relation to the daily caloric intake of approximately 2000kcal, this result is practically meaningless, as it represents less than 1% (0,3%) of the total calories we eat.
Some calculations indicate that for us to lose weight 1 kg of fat, it is necessary to consume about 7500kcal performing physical activities or reducing caloric intake. To achieve the same feat with the ingestion of cold water (at 4 ºC), it would be necessary to consume about 220 liters - a quantity impractical, great even for the period of a month. So there are more viable strategies to lose fat, such as decrease of calorie intake and the regular practice of physical exercises. It is worth mentioning that this does not imply reducing water consumption, since it is extremely important for the entire metabolism of the body.
By Rafael Hellerbrock
Graduated in Physics
Source: Brazil School - https://brasilescola.uol.com.br/fisica/agua-gelada-ajuda-emagrecer.htm
