Since antiquity, man has been interested in answering the question about the constitution of matter.
Several personalities from the scientific community took a stand on the subject, but it was only at the end of the 19th century that the mysteries about atomic composition began to be unraveled.
The ancient philosopher Leucippus, for example, believed that the atom was made up of tiny particles.
After this definition, it was proposed by Democritus and Dalton that atomic particles were massive and indivisible.
This fact was later disputed by Thomson, an English physicist who discovered the existence of the electron and was able, then, to confirm the idea previously proposed by Leucipo.
After this discovery, Thomson presented a model in which the atom was formed by a sphere of positive charges and inside the nucleus there were electrons.
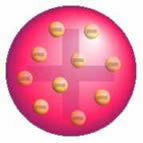
Thomson's Atom Model - In a positively charged, uniformly distributed mass sphere,
they would be encrusted with negatively charged electrons, as in a pudding.
Even after Thomson's theory, the atomic model still had a theoretical-experimental deficiency, capable of proving its veracity.
The subject was once again warmly discussed when physicist Rutherford's team observed that there was a very large deviation (and inexplicable, according to the raisin pudding theory) when some radioactive alpha particles passed through a thin layer of lamina metallic.
After analyzing and studying the fact, Rutherford came to the conclusion that the radius of the atom is 10,000 times larger than the radius of the nucleus.
Through all the study of the atom, Rutherford and his team arrived, in 1911, at an atomic model, which they called the planetary model of the atom.

In 1913, Niels Bohr deciphered the atomic model, applying the quantum of action discovered by Planck to his studies. The quantum of action was, in fact, the great wildcard that would come to complete and clarify the atomic model.
Bohr efficiently and simply adjusted the model presented by Rutherford reaching the following conclusion:
The electron acquires energy, which is represented through a defined orbit. The allowed orbits form the energy levels.

By Talita A. angels
Graduated in Physics
Brazil School Team
Physics Modern - Physics - Brazil School
Source: Brazil School - https://brasilescola.uol.com.br/fisica/do-atomo-rutherford-ao-atomo-bohr.htm