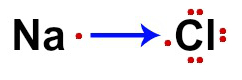
An important concept in the study of Optical Isomerism is that of polarized light. It is necessary for the student to understand what it is, because compounds are only considered optical isomers if they deviate from the plane of vibration of polarized light. So let's see its concept and how it is obtained:
White light, like sunlight and incandescent light, is a type of light no polarized, because when it is emitted, in general, its rays propagate in all planes.
This is because white light is made up of electromagnetic waves that vibrate in infinite planes perpendicular to the direction of light propagation. This natural light is called polychromatic and is usually represented as follows:
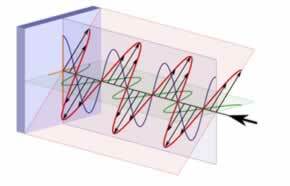

already the polarized lightis one that varies in only one plane and can be represented by:

Do not stop now... There's more after the advertising ;)
Polarized light can be obtained by passing natural light through a polarizer or by a polarizing substance. Some examples are a polarizing lens or a Nicol prism.
This polarized light is used to study the optical activity of organic compounds. When it crosses a certain substance, one of the three situations below can occur:
1. The vibration of the plane of polarized light is shifted to the right: This means that the compound is optically active and it constitutes the optical isomer called right-handed;
2. The vibration of the plane of polarized light is shifted to the left: This means that the compound is optically active and it constitutes the optical isomer called levogyro;
3. The vibration of the plane of polarized light is not diverted: This means the compound is optically inactive. It can be a racemic mixture (50% right-handed and 50% left-handed) or it can be a substance that has no optical activity.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Polarized Light"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/luz-polarizada.htm. Accessed on June 27, 2021.