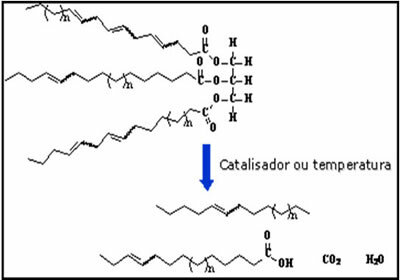
At chemical formulas are representations used to indicate which chemical elements are part of the substance's composition and also to represent the type of interaction between the participating atoms.
Through the analysis of the formula of a chemical substance, we can determine the nature of the chemical bonds (ionic, covalent or metallic) that formed it, as well as its physicochemical behavior. Some of the properties that are related to the physicochemical behavior of a substance are:
Solubility
Fusion point
Boiling point
Reactivity
Now know the types of compounds that make up chemicals and also the formulas that represent them.
Types of compounds
The) ionic compounds
Ionic compounds are all chemical substances formed by ionic bonding between atoms, that is, in these compounds, there are atoms that lose and atoms that gain electrons. The combination of chemical elements that form this type of compound can be:
Metal with ametal
Metal with hydrogen
b) Covalent compounds
Covalent compounds are all chemical substances formed by covalent bonding between atoms, that is, in these compounds, there are atoms that share electrons with each other. The combination of chemical elements that form this type of compound can be:
ametal to ametal
Ametal with hydrogen
hydrogen with hydrogen
c) Metal compounds
Metal compounds are all chemical substances formed by atoms of a single metallic element. In these compounds, atoms only share electrons with each other.
Formulas for ionic compounds
The) ion-formula
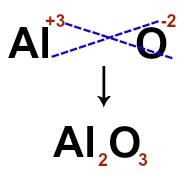
The formula ion indicates the number of atoms that form the ionic unit of the compound, as in the case of chloride of sodium, whose formula ion is NaCl. In the formula, we identify the presence of a sodium atom and an atom of chlorine.
To build the ion-formula, just cross the charges of each of the ions that make up the ionic compound. For the Al cation+3 and the anion O-2, for example, when we cross the charges and disregard the signals, we have the following formula ion:
b) Electronic formula
The electronic formula is used to represent the loss and gain of electrons of the atoms involved in the formation of the compound. Around the abbreviation of each element, we have each of the electrons of the valence layers.
In the case of sodium chloride (NaCl), in Na we have only one valence electron (because it is from the IA family) and, in Cl, we have seven valence electrons (because it is from the VIIA family) represented.
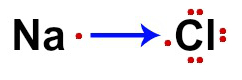
NaCl electronic formula
The gain of an electron by Cl (nonmetal) and the loss of an electron by Na (nonmetal) are represented by an arrow. According to the octet theory, Na loses an electron for having only one, and Cl gains one to complete eight valence electrons.
Do not stop now... There's more after the advertising ;)
For covalent compounds
a) Molecular formula
The molecular formula is used to represent, in a simplified form, a covalent substance. The molecular formula of water, which is H2O, for example, has two hydrogen atoms and one oxygen atom.
Unlike the ion-formula of ionic compounds, the molecular formula of a covalent compound is not built with as much simplicity, as different amounts of atoms of the same elements form different substances, such as water (H2O) and hydrogen peroxide (H2O2).
It is very common, in exercises, the molecular formula:
Be provided by the exercise itself;
Be determined through a calculation;
Be determined by counting each element from the structural formula;
Be determined from the substance name.
b) Structural formula
The structural formula of a covalent substance is used to represent the number of bonds each of the atoms make in the molecule. The links that are used in the structural formula are:
Single link: represented by a dash (─), indicates a single link;
Double bond: represented by two dashes (=), indicates two bonds;
Triple bond: represented by three dashes (≡), indicates three bonds;
dative link: represented by an arrow (→), indicates a single connection.
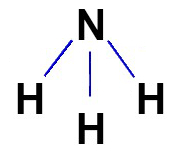
The structural formula of the covalent compounds should be represented, whenever possible, according to the molecular geometry of the molecule in question. See the representation of the structural formula of ammonia, which has pyramidal geometry:
c) Electronic formula
The electronic formula for a covalent compound demonstrates the sharing of electrons between the atoms that make up the molecule. Instead of the dashes used in the structural formula, we have the use of spheres to represent the electrons shared between atoms. Look:
Single bond: sharing two electrons (one from each of the atoms involved);
Double bond: sharing four electrons (two from each of the atoms involved);
Triple bond: sharing six electrons (three from each of the atoms involved);
Dative bond: sharing of two electrons (both being from a single atom between those involved),
Thus, for ammonia, whose structural formula was represented above, its electronic formula is:
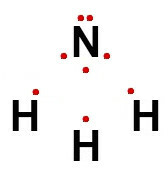
For metallic compounds
Metal compounds, as they are formed exclusively of atoms of a single metal, have as their chemical formula the abbreviation of the chemical element:
Copper substance: Cu
Gold substance: Au
Iron substance: Fe
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "Chemical substance formulas"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/formulas-substancias-quimicas.htm. Accessed on June 27, 2021.