In this text, we will talk about some separation methods, for heterogeneous mixtures, that can be adopted both in industries and in the kitchen at home. Are they: fractional dissolution, filtration and decant.
To separate the components of a Mix, different methods are used, which differ in terms of type of mixture (homogeneous and heterogeneous) how about number of components and yours physical states (solid, liquid and gas).
According to the complexity of the process, these mixture separation methods are only adopted in industries and laboratories, but many of them can be done at home, with simple utensils, such as coffee filters, sieves, funnels, etc.

Also know: Picking, flotation and levigating
fractional dissolution
Fractional dissolution is a method employed to separate a heterogeneous mixture from two or more solids, being that only one of them is soluble in a given solvent. Therefore, to use this procedure, it is necessary to have knowledge of the mixture components and their respective solubilities, in order to use the appropriate solvent.
Fractional dissolution can be used to separate a mixture between sand and table salt, for example. When adding water, only the salt will be solubilized by the water, and the sand will form a solid phase, which can be separated from the liquid phase (water + table salt) using a simple filter.
It is important to emphasize that this method needs other auxiliary methods to complete the separation of the mixture components. Fractional dissolution is used only to start the whole process.
- Where is used
Fractional dissolution is mainly used in chemical analysis laboratories is on manufacturing processes, in which it is necessary to fractionate and purify solids present in mixtures.
- Industrial application
Fractional dissolution is applied in determining the alcohol content present in Gasoline commercial. When adding water to the mixture of gasoline and alcohol, as it has greater interaction with water, the alcohol separates from the gasoline and forms a single aqueous phase, leaving only gasoline in the other phase. This makes it possible to calculate whether the amount of alcohol present in the gasoline sold is within the parameters established by law.
Also know: what is an alcohol?
filtration
Filtration is a mixture separation method that makes use of a filter to separate the components of a mixture, which can be of the solid + liquid (sand + water) or solid + gas (dust + air).
According to the components of the mixture, we can choose between two filtration methods: simple and vacuum, in which the air is removed from the container that will collect the liquid.
- Simple filtration: the mixture is poured over the filter, which will retain the solid.
- Vacuum filtration: the process is similar to simple filtration, but it becomes faster because the system is coupled to a vacuum pump, which will remove the air from the collecting container, sucking the filtrate through the vacuum.

- Occupation
Regardless of the type of filtration adopted, the purpose of this method is to separate the solid from the other phase, passing the mixture through a filtering medium, which will retain the solid, and the liquid or gaseous part, called filtered, is collected in its own container.
Such method is used in order to obtain a filtrate free of solid particles, or in laboratory analyses, in stations of sewage treatment, or in industries. In our daily life, filtration is present in the preparation of coffees, teas, in the use of vacuum cleaners, swimming pool filters, among others.
- Used equipments
In simple filtration, the equipment used are:
- Funnel
- Collector bottle (usually Erlenmeyer or beaker)
- filter paper
- Universal support (to hold the funnel)

In vacuum filtration, the following equipment is used:
- Buchner's Funnel
- Kitassate
- filter paper
- Pump vacuum

Decantation
Decantation is a method used to separate mixtures such as: solids and liquids that form two phases, that is, the solid is not dissolved in the liquid; and still, immiscible liquids, that is, that they are not solubilized.
In addition, it is necessary that the components have difference of density, so that, when left to rest, one of them goes to the container bottom, and the other float.
- How is it made?
For mixtures formed by solids and liquids, just put the mixture in a container and let it rest until, due to the difference in density, the solid is all at the bottom of the container.
After this process, it is necessary to apply the siphoning method to remove the liquid that is at the top of the mixture, in order to complete the separation of the two components.
now stop mixtures formed of two liquids, we use a device called bromine funnel or decanting funnel. Inside the funnel, we place and let the mixture rest, until the liquid with higher density goes completely to the bottom of the funnel and, consequently, the one with lower density is at the top.
After that, we open the valve of the bromine funnel so that the denser liquid flows and is collected in some other container, such as a beaker.
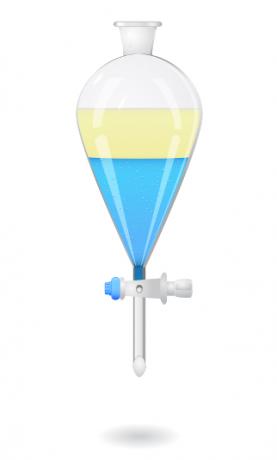
- Decantation in the industry
As with other methods of separation of mixtures, decantation is applied in industrial processes, such as in dairy industries to separate the cream from the milk, and in the winemaking, in which, after the process of fermentation, the yeast is separated from the wine by decanting.
Water and sewage treatment plants also use the process of sedimentation at various stages of the decanting process, in order to remove impurities present in water streams. If you are more interested in this subject, read our text: Decantation.

By Victor Ferreira
Chemistry teacher
Source: Brazil School - https://brasilescola.uol.com.br/quimica/dissolucao-filtracao-decantacao.htm
