Acids and bases are two chemical functions that are considered opposites., this is because their properties are usually inverse. For example, if we consider foods present in our daily lives that are acidic, we will see that their taste, in general, is sour, as occurs with lemon. However, foods that are basic have an astringent taste (which “binds” the mouth), like a green banana.
But identifying a substance as acidic or basic just by taste, as well as being a method that has many chances of failing, it is also highly dangerous as there are many acids and bases that are strong, toxic and can even kill, such as acid. sulfuric (H2ONLY4), used in automobile batteries, and sodium hydroxide (NaOH), commercially known as caustic soda.
Thus, organoleptic properties (properties that concern our senses, such as taste and smell) are not the ones used to identify acids and bases. Note below other properties of these organic functions that serve to compare and distinguish them:
- Solubility in water:
You acids usually be well soluble in water, while most bases é insoluble.
Alkali metal bases are soluble, alkaline earth metal bases are poorly soluble and bases from other metals are insoluble (an exception is ammonium hydroxide, NH4OH, which exists only in aqueous solution, bubbling the ammonia gas in water and is therefore soluble in it).When we say “insoluble”, we mean that these substances are practically insoluble, because no substance is completely insoluble in water.
- Structure:
All the acids are molecular, that is, formed by covalent bonds in which electrons are shared. An example is hydrochloric gas, which is formed by sharing a pair of electrons between hydrogen and chlorine:

already the bases can be ionic or molecular. Those that have the alkali and alkaline earth metals are ionic, and the others are molecular.
Examples:
NaOH: ionic base formed by Na ions+ and oh-;
NH4OH: molecular base of ammonia in water.
Do not stop now... There's more after the advertising ;)
- Electric conductivity:
All acids only conduct electrical current when they are dissolved in water, because when they are in an aqueous medium, they undergo ionization, that is, they release ions.
Example:
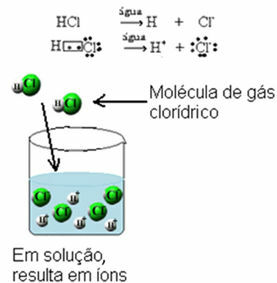
All the bases also conduct electric current in solution., as the ionic ones undergo dissociation (release the ions already existing in the formula) and the molecular ones undergo ionization, reacting with water and releasing ions. At Alkali metal bases also conduct electric current when in a liquid (molten) state.
- Action on indicators:
You acid-base indicators are natural or synthetic substances that undergo a change in color when they come into contact with an acid or a base. If an acid causes the indicator to change color, the base will return the indicator to its original color and vice versa.
For example, phenolphthalein is a widely used acid-base indicator, and in a basic medium it becomes a very intense pink; already in an acidic medium, it becomes colorless. Litmus paper is also a good indicator, as in an acid it turns red; and on one base, it turns blue.
This also serves to indicate the pH difference that exists between acids and bases.
- PH:
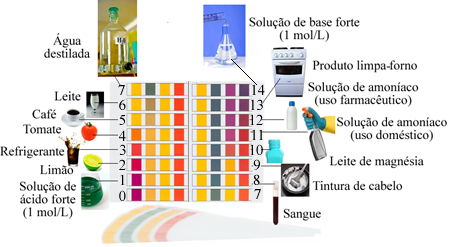
A medium considered neutral has a pH equal to 7, as is the case with distilled water.
Acids have a pH of less than 7, While the bases have a pH greater than 7.
Examples of solutions with a pH close to that indicated by the scale:
- Reciprocal action:
When placed in contact, acids and bases react with each other, neutralizing each other, that is, making the pH of the medium neutral. This is because the H cation+ from the acid reacts with the OH anion- from the base, forming water. This type of reaction is called a neutralization reaction and it also produces a salt.
By Jennifer Fogaça
Graduated in Chemistry