carbon dioxide or carbon dioxide (CO2) is known as an atmospheric pollutant. However, its occurrence is natural and necessary because it is one of the gases that cause the greenhouse effect that keeps the temperature of our planet pleasant.
Unfortunately, especially from the Industrial Revolution, the concentration of carbon dioxide in the atmosphere has been growing more and more. This gas is one of the products of burning fossil fuels, such as gasoline, diesel oil and coal. So, the greenhouse effect has been intensified, generating global warming.
But scientists of english company Air Fuel Synthesis stated that they achieved the feat of transforming carbon dioxide into gasoline. Ever wonder? In addition to removing the CO2 of the atmosphere, reducing pollution and global warming, on top of that they get a new source of fuel! This news that scientists have managed to turn pollution into fuel is really interesting, to say the least, don't you think?!
But how do these scientists manage to turn carbon dioxide into gasoline?
Simply put, they collect atmospheric air through pipes, reacting it with sodium hydroxide to separate carbon dioxide. When passing electrical current through the CO2, it is broken and carbon is obtained, which, in turn, is put to react with hydrogen gas (H2).But where did this hydrogen gas come from? It came from the electrolysis of water, which is a process in which an electric current is passed through water, leading to its decomposition, that is, to the separation of the gases that compose it, which are hydrogen gas and oxygen.
2 hours2O → 2 H2 + 1 O2
In the mini reactor, carbon and hydrogen generate Hydrocarbons that make up the Gasoline (mixture of hydrocarbons having 6 to 10 carbon atoms).
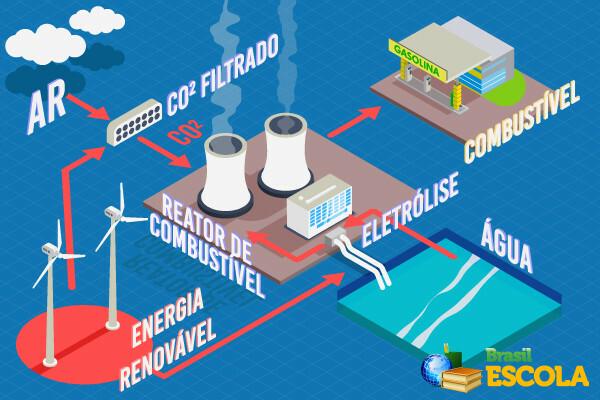
Another reason that drives this transformation of pollution into fuel is the fact that fossil fuels (mainly petroleum derivatives) are non-renewable and generate pollution not only in their burning, but also in their exploration and extraction. With the discovery of this new technique, this would not happen, because, despite its use releasing carbon dioxide, in its production process this gas is removed from the atmosphere. Thus, there would be no significant contributions to the amount of carbon in the atmosphere and, consequently, there would be no intensification of the greenhouse effect or global warming.
The only obstacle so far is the economic factor, as inventors admit that the price of gasoline produced on a small scale is not competitive, being R$ 20.00 per liter. But with investments for large-scale productions, it is possible that the process will have its costs reduced. This requires an investment of around US$ 2 million. There are already entrepreneurs willing to invest in this process.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/cientistas-conseguem-transformar-gas-carbonico-gasolina.htm

