happens to transformationisochoric when a given mass of a gas undergoes changes in temperature that trigger pressure changes or vice versa, considering that temperature and pressure, in this case, are directly proportional quantities, while the system volume remains unchanged.
See too: What is partial pressure of gases?
What is an isochoric transformation?
An isochoric transformation is when a change in temperature and pressure happens to a given mass of gas, but its volume remains the same. The pressure change will be directly proportional to the temperature, that is, if a gas, in a certain closed system, doubles its absolute temperature, this will cause an increase in pressure to twice what it was initially.
This type of transformation is also called isovolumetric or isometric. The isochoric term comes from the Greek Isokhora, on what iso means "equal" and khora means “place or volume” (in this context).
Examples:
The tires of a car undergo isochoric transformation, we can observe this when we make its
calibration. On hot days, the pressure given on the calibration monitor is higher than on cold days. This is because, with an increase in temperature, the pressure increases, as the volume remains.
Other examples are the spray deodorants, in this type of product there is a warning on the label not to be stored or subjected to high temperatures, because, in addition to some of its substances being flammable, there is the condition of isochoric transformation or isovolumetric. As the aerosol deodorants are inside a sealed bottle and in a gaseous state, the an increase in temperature will lead to an increase in pressure and the risk of an explosion.
Characteristics of an isochoric transformation
- Constant volume.
- Pressure and temperature are directly proportional.
These characteristics can be described by the following formulas:

Graph of an isochoric function
Note the following table which describes the behavior of a gas in relation to pressure, temperature and the resulting constant.
Temperature (°C) |
Pressure (atm) |
P/T = constant |
50 |
5 |
10 |
100 |
10 |
10 |
150 |
15 |
10 |
200 |
20 |
10 |
Note that pressure follows temperature rise so that the P/T ratio remains constant, meaning that pressure and temperature are directly proportional quantities. Therefore, the graph describing isochoric transformations is of linear type. Look:

Who discovered the isochoric transformation?
Jacques Alexandre César Charles (1746-1823) was the French scientist who, studying the behavior of gases in a closed system for the creation of a precision thermometer, concluded the studies on isochoric transformations.
The scientist Joseph Louis Gay-Lussac (1778-1850) also developed a study on expansion and shrinkage of a gaseous system. These were independent analyses, but because they reached the same conclusion, they divided the credits. Today some authors refer to the last two physical laws of gases that explain the isobaric transformations and isochoric like 1st and 2nd laws of Charles-Gay-Lussac.
See too: Gay-Lussac volumetric law
Exercisesresolved
Question 1 - (PUC-RJ) A bicycle tire is calibrated at a pressure of 4 atm, on a cold day, at a temperature of 7 °C. The volume and amount of injected gas are the same. What will the calibration pressure be in the tire when the temperature reaches 37°C?
a) 21.1 atm
b) 4.4 atm
c) 0.9 atm
d) 760 mmHg
e) 2.2 atm
Resolution
Alternative A
1st step: identify the system and extract the data.
Isovolumetric system
P1 = 4 atm
T1 = 7°C
T2 = 37°C
P2 = ?
Using the formula:
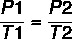
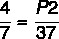
7 x P2 = 37 x 4
P2 = 148 / 7
P2 = 21.14 atm
Question 2 - (Unifor-CE) Examine the figure below.
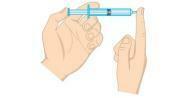
The gas pressure inside the syringe can be decreased:
a) placing the syringe in ice water, keeping the end capped.
b) squeezing the plunger, keeping the end capped.
c) placing the syringe in hot water, keeping the end capped.
d) opening the tip and expelling half of the air out of the syringe.
e) pulling the plunger, keeping the end open.
Resolution
Alternative A. The pressure of a gas is directly proportional to the temperature in an isochoric system, that is, without changing the volume or mass of the gas. When there is a decrease in temperature, there will also be a drop in pressure.
By Laysa Bernardes Marques de Araujo
Chemistry teacher
Source: Brazil School - https://brasilescola.uol.com.br/quimica/transformacao-isocorica.htm
