The official nomenclature of the ethers, according to IUPAC, can be done in two ways. See each one:
1st way:

Examples:
CH3 — O — CH2 — CH3→ metoxyetanO
CH3 — CH2 — O — CH2 —CH3→ etoxyetanO
CH3 — CH2 — O — CH2— CH2 —CH3→etoxypropanO
CH3 — O — CH2— CH═CH —CH3→ metoxybutenO
CH3 — CH2 — CH2 — O — CH2— CH2 — CH2 — CH2 — CH3→propoxypentanO
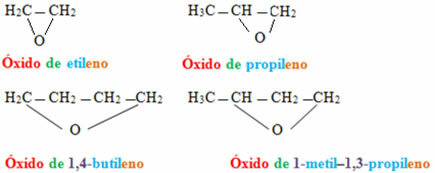
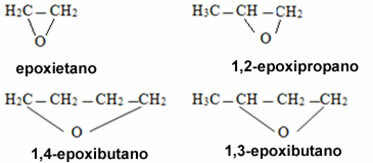
If they are ethers of closed chains, the nomenclature will be different:

Examples:
Do not stop now... There's more after the advertising ;)
2nd way:
The two groups linked to oxygen are considered as substituents, being indicated in order of complexity with the suffix ich, that is, it follows the following rule:
ether + 1st group + 2nd group + ich
These groups should appear and
Examples:
CH3— O —CH2 —CH3→ ether ethylich and methylich
CH3 —CH2—O —CH2 —CH3→etherdiethylich
CH3 —CH2—O —CH2—CH2 —CH3→etherethylich and propileich
CH3 —CH2 —CH2—O —CH2—CH2 —CH2 —CH2 —CH3→etherpropileich and pentylich
In the case of cyclic compounds, they are called epoxides:
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Nomenclature of the Ethers"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/nomenclatura-dos-Eteres.htm. Accessed on June 27, 2021.
Base Nomenclature, Aqueous Solution, Ionic Dissociation, Cation, Anion, Sodium Hydroxide, Aluminum Hydroxide, Iron Hydroxide, Copper Hydroxide, Ferric Hydroxide, Calcium Hydroxide.