Electron distribution or electron configuration the way chemical elements are ordered considering the number of electrons they have and their proximity to the atomic nucleus.
Electronic tiered distribution
After several atomic models emerged, the Bohr model suggested organizing the electrosphere into orbits.
The electrons are organized and distributed through the electronic layers, some being closer to the nucleus and others farther away.
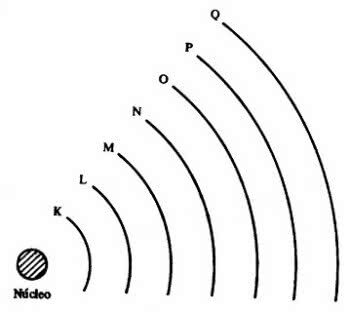
Then came the 7 electronic layers (K, L, M, N, O, P and Q), which are represented by the horizontal lines numbered 1 to 7 in the periodic table.
Elements on the same lines have the same maximum number of electrons and also the same energy levels.
Thus, it is possible to observe that electrons are in energy levels and sub-levels. So each has a certain amount of energy.
|
Energy level |
Electronic Layer |
Maximum Number of Electrons |
|---|---|---|
| 1° | K | 2 |
| 2° | L | 8 |
| 3° | M | 18 |
| 4° | N | 32 |
| 5° | O | 32 |
| 6° | P | 18 |
| 7° | Q | 8 |
THE valence layer it is the last electronic layer, that is, the outermost layer of the atom. According to
Octet Rule, atoms have a tendency to stabilize and become neutral.This happens when they have the same amount of protons and neutrons, with eight electrons in the last electron shell.
Later, the energy sublevels appeared, represented by the lowercase letters s, p, d, f. Each sublevel supports a maximum number of electrons:
| sublevels | Maximum number of electrons |
|---|---|
| s | 2 |
| P | 6 |
| d | 10 |
| f | 14 |
Pauling diagram
The American chemist Linus Carl Pauling (1901-1994) studied atomic structures and developed a scheme that is still used today.
Pauling found a way to put all the energy sublevels in ascending order, using the diagonal direction. The scheme became known as the Pauling diagram.
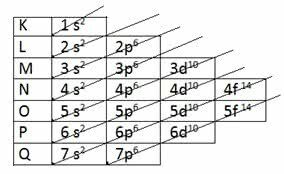
Ascending order: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f14 6d10 7p6
Note that the number indicated in front of the energy sublevel corresponds to the energy level.
For example, in 1s2:
- s indicates the energy sub-level
- 1 indicates the first level, located on layer K
- exponent 2 indicates the number of electrons in this sublevel
How to do electronic distribution?
To better understand the electronic distribution process, look at the solved exercise below.
1. Make the electronic distribution of the element Iron (Fe) which has atomic number 26 (Z = 26):
When applying the Linus Pauling Diagram, the diagonals are traversed in the direction indicated in the model. The energy sub-levels are filled with the maximum number of electrons per electron shell, until completing the element's 26 electrons.
To make the distribution, pay attention to the total number of electrons in each sublevel and in the respective electronic layers:
K - s2
L - 2s2 2p6
M - 3s2 3p6 3d10
N - 4s2
Note that it was not necessary to do the electronic distribution in all layers, since the atomic number of Iron is 26.
Thus, the electronic distribution of this element is represented as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d6. The sum of the exponent numbers totals 26, that is, the total number of electrons present in the Iron atom.
If the electronic distribution is indicated by layers, it is represented as follows: K = 2; L = 8; M = 14; N = 2.
Take the opportunity to test your knowledge in Exercises on Electronic Distribution.
At periodic table, this is shown as follows:

Read too:
- Electronic Affinity
- Quantum Numbers
- Exercises on the Periodic Table
- Exercises on Organizing the Periodic Table