Cells, or electrochemical cells, and batteries are devices in which chemical energy is spontaneously transformed into electrical energy.The cell is made up of just two electrodes and an electrolyte, while the battery is a set of batteries in series or in parallel.
At primary batteries are non-rechargeable devices, being that when the oxidation-reduction reaction that takes place inside them ceases, they must be discarded.
For each equipment, a type of battery is indicated and, among the primary batteries currently used, the main ones are: Leclanché dry cells (common batteries or acid cells), alkaline batteries and lithium/manganese dioxide batteries.
See what sets them apart and what equipment they are suitable for:
- Leclanché Dry Cells:
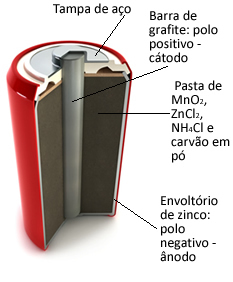
These piles are basically formed by a zinc envelope, separated by a porous paper and a central graphite bar surrounded by manganese dioxide (MnO2), powdered charcoal (C) and a wet paste containing ammonium chloride (NH4Cl), zinc chloride (ZnCl2) and water (H2O).
O zinc works like the anode, losing electrons; it's the graphite works like the cathode, conducting electrons to manganese dioxide:
Anode Half Reaction: Zn (s) → Zn2+(here) + 2 and-
Cathode Semi-Reaction: 2 MnO2(aq) + 2 NH41+(here) + 2e- → 1 Mn2O3(s) + 2NH3(g) + 1 hour2O(1)
Global Reaction: Zn (s) + 2 MnO2(aq) + 2 NH41+(here) → Zn2+(here) + 1 Mn2O3(s) + 2NH3(g)
This type of battery is suitable for equipment that requires light and continuous discharge, such as remote control, wall clock, portable radio and toys.
You can get more details about the origin, functioning, durability, ddp, hazards and precautions that must be taken with these batteries in the text “Leclanché Dry Stack”.
Do not stop now... There's more after the advertising ;)
- Alcaline batteries:
Its operation is very similar to that of Leclanché dry cells, however, the only difference is that in place of chloride ammonium (which is an acidic salt), a strong base is added, mainly sodium hydroxide (NaOH) or potassium hydroxide (KOH).
Anode Half Reaction: Zn + 2 OH → ZnO + H2O + 2e-
Cathode Semi-Reaction: 2 MnO2 + H2O + 2e-→ Mn2O3 + 2 OH
Overall reaction: Zn +2 MnO2→ ZnO + Mn 2O3

Alkaline batteries are more advantageous than acid ones in the sense that they have a greater durability, in typically provide 50-100% more power than an ordinary battery of the same size, plus there's less danger of leaks.
They are mainly indicated for devices that require fast and more intense downloads, such as radios, portable CD/DVD and MP3 players, flashlights, digital cameras, etc.
Also read the text Alcaline batteries.
- Lithium/manganese dioxide batteries:
These batteries are lightweight and generate a large voltage (about 3.4 V), which is why they are often used in small equipment such as watches and calculators. Unlike the previous cases, its format is currency, as shown in the following image:

The anode is lithium, the cathode is manganese dioxide and the electrolyte is a saline solution:
Anode Half Reaction: read →read+ + and−
Cathode Semi-Reaction: MnO2 + read+ + and−→MnO2(li)
Overall reaction: Li + MnO2 → MnO2(li)
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Most Common Types of Cells and Primary Batteries"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/tipos-pilhas-baterias-primarias-mais-comuns.htm. Accessed on June 27, 2021.


