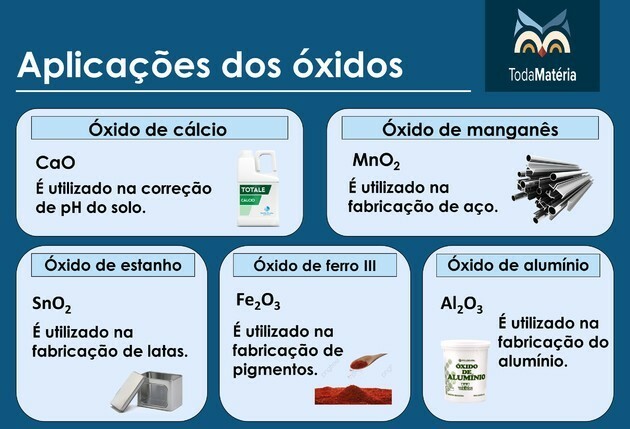
THE density is a quantity that relates the mass and volume of a given material.
It is given by the formula: density = pasta or d = m
volume v
The unit of density in the International System is kg/m3, but in everyday calculations the following units are also used: g/cm3, g/ml and g/L.
Thus, when calculating the density of water, for example, it is enough to place it in a beaker and measure its volume. Then we weigh this beaker with the water and reduce it by the mass of the beaker, discovering the mass of the water. Then, just divide it by the volume it occupies.
For regular solids such as a cube, measuring the mass is also easy, as you just put it on a scale. The volume follows specific mathematical formulas that only need to be calculated. For example, in the case of a cube, just multiply its height by its length and width to find its volume. Then we substituted it in the density formula.
However, when it comes to irregular solids (which do not have a defined geometric shape), there are no mathematical formulas to calculate their volume. So, the question arises:
how to calculate the density of irregular solids?The Greek mathematician Archimedes was faced with this question in the third century BC. Ç. The story goes that the king ordered him to find out if his crown was really made of gold. Archimedes knew that it was enough to simply compare the density of the crown with the density of gold, as density is an intensive property, that is, it does not depend on the quantity of the sample. The density of an ice cube, for example, is exactly the same as that of a iceberg. But the problem was to determine the volume and, consequently, the density of the crown, since it did not have a regular shape.
One day, when he got into a bathtub to take a shower, Archimedes noticed that the water level was rising. At that moment he had a brilliant idea and was so excited that he ran naked through the streets and shouting the famous word:Eureka!
Do not stop now... There's more after the advertising ;)

Illustration of Archimedes and his discovery
Thus was discovered the Archimedes' Principle, which is used to measure the volume of irregular solids. This principle suggests the following: we measure a certain amount of water in a beaker, then add the irregular solid and observe the change in volume that the water has undergone, and that's it: this change in the volume of water is exactly the volume of the solid!

Archimedes' Principle is used to measure the volume of irregular solids
If it becomes easier, instead of simply looking in the beaker measurement for the change in volume, you can use a tube of overflow, which has a part that allows water to flow out of the cylinder when the irregular object is added. Leaving water is measured separately.
Note in the figure above that Archimedes measured the volume of a bar of gold pure that had the same mass as the crown and calculated its density (19.3 g/mL or g/cm3) and did the same with the crown. Note that the volume of water and therefore the volume of the crown and the gold bar were not the same. This meant that, in reality, the crown was not pure gold, it was actually a alloy.
Even today this method is used to identify precious materials such as jewelry. If the density of a jewel made with a metallic alloy is above 14g/mL, this indicates that it is a gold piece of at least 18 carats, made of about 75% gold, 13% silver and 12% copper.
*Image with copyright: nephthali / Shutterstock.com.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Calculation of density of irregular solids"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/calculo-densidade-solidos-irregulares.htm. Accessed on June 27, 2021.
Chemistry

Properties of gold, characteristics of gold, precious metal, 24 carat gold, process for obtaining precious jewels, gold and silver metal alloy, electric current for gold testing, tin and sulfur alloy, connection metallic.