Addition reactions are those in which a reactant is added to an organic molecule.
They occur mainly in unsaturated compounds, especially alkenes, and occur as shown generally below, where the double or triple bond is broken. In fact, the pi link (π), which is weaker than the sigma link (σ) of the double, is broken, allowing the addition of atoms or groups of atoms to the carbons that participated in the unsaturation.
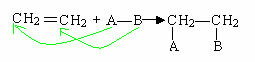
The most common addition reactions are:
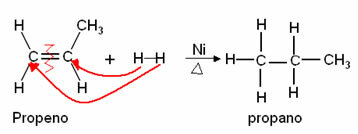
1. Addition of Hydrogen (Hydrogenation or Sabatier-Senderens reaction): Reaction with hydrogen gas (H2), metal catalyzed. If the reaction is an alkene, it produces an alkane:
2. Addition of Halogens (Halogenation): Halogens are the elements of the 17A family of the Periodic Table. However, of these, the most used are: Cl2 and Br2. In this case, there is the formation of an alkyl dialect:
Do not stop now... There's more after the advertising ;)

3. Addition of Halogenhydrides (Hydro-Halogenation): A halide (HX) is a compound in which a halogen is bonded to hydrogen, eg the hydrogen chloride from the reaction below, with formation of an alkyl halide:
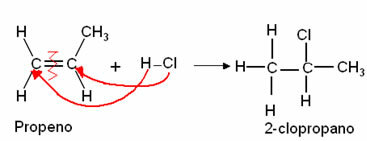
It is important to remember that this and the next reaction follow Markovnikov regiochemistry, in which Hydrogen enters the most hydrogenated carbon.*
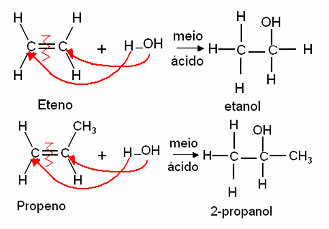
4. adding water: This hydration reaction takes place in an acidic medium as a catalyst and an alcohol is formed. With the exception of ethylene, the other alkenes only give rise to secondary alcohols in this reaction:
* For a more detailed explanation of Markovnikov Regiochemistry, read the text “Markovnikov's Rule” .
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Additional Reactions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/reacoes-adicao.htm. Accessed on June 27, 2021.