THE enthalpy (H) it can be defined as the energy content of each material or as the overall energy of a system. However, it is not possible, in practice, to determine the enthalpy of a substance. So, what is usually measured is the enthalpy variation 

We will analyze here the variation of enthalpy in exothermic reactions (the word “exo” comes from the Greek and means “out, out of, out”), which are those reactions in which there is heat release. In everyday life, there are many examples of these reactions, such as combustion and the mixture of quicklime with water.
In these cases, the enthalpy variation 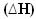 will be exactly the amount of heat released, given by the value of the final enthalpy minus the initial enthalpy (Hf - Hi) or, as it is a reaction, the enthalpy value of the products minus that of the reactants (HP - HR).
will be exactly the amount of heat released, given by the value of the final enthalpy minus the initial enthalpy (Hf - Hi) or, as it is a reaction, the enthalpy value of the products minus that of the reactants (HP - HR).
As heat is released, energy is lost. Thus, the total energy of the system decreases, so that the enthalpy of the products will always be lower than the of the reagents, so the enthalpy variation in exothermic reactions will always be negative, less than zero 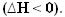
Do not stop now... There's more after the advertising ;)
Considering a generic exothermic reaction:
| A → B + heat |
This can be expressed using an enthalpy diagram like the one below:
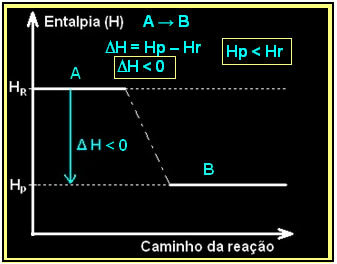
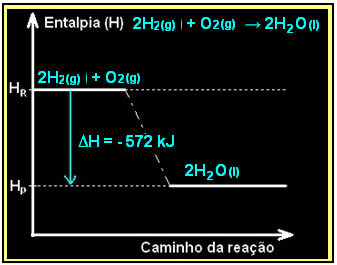
The combustion of hydrogen gas, with the formation of liquid water, takes place with the release of energy. The value of the enthalpy variation of the system in this reaction is given by:
2h2(g) + O2(g) → 2H2O(1)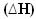 = - 572 kJ
= - 572 kJ
Your diagram would be represented as follows:
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Variation of Enthalpy in Exothermic Reactions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/variacao-entalpia-reacoes-exotermicas.htm. Accessed on June 28, 2021.
Chemistry

Thermochemistry, Enthalpy, Released Heat, Exothermic Reaction, Combustion Reaction, External Environment, Endothermic Reaction, Reaction chemistry, energy exchange, reagents, light emission, light absorption, heat, electricity, components, physical state, products.