active isomers or optically active isomers (IOA) are molecules of a certain chemical substance that can carry out the polarization and deviation of the plan of light right or left. To check whether or not a substance has active isomers, just check if there is an asymmetric carbon in its structural formula:

Analyzing the chain represented in the image above, we can see that the central carbon has four different ligands (OH, H,CH3 and Cl), which makes it a carbon asymmetric, also called carbon chiral. Thus, whenever a chain has one or more chiral carbons, there will be active isomers.
You types of active isomers(IOA) they are:
Right-handed: Active optical isomer that bends polarized light to the right;
Levogiro: Active optical isomer that bends polarized light to the left.
Observation: The presence of asymmetric carbon in a structural formula indicates that the substance must have the levorotatory isomer and the dextrorotatory isomer. It is not up to us to know which way the light was deflected, as the presence of the chiral carbon already evidences this fact. Always half of the existing molecules are left-handed and the other half are right-handed.
Left-handed and right-handed molecules of any organic substance always have the same physical properties (point of melting, boiling point, density, solubility etc.), but present chemical activities (behavior in the organism) many different. An example is adrenaline. Only the adrenaline levorotatory molecule acts in the body, while the dextrorotatory molecule does not.
See now the structural formula of the butan-2-ol substance to check whether or not it has active isomers:
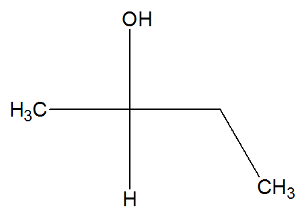
We can observe, in the structure of butan-2-ol, that carbon 2 has four different binders (OH, H,CH3, CH2-CH3), so it is a chiral carbon and presents a dextrorotatory active isomer and another levorotary active isomer.
Calculation of active isomers (IOA)
The Dutch Chemist Jacobus Henricus van't Hoff developed a formula that allows the calculation of how many active isomers (IOA) a given substance can present. Look:
IOA: 2no
n: is the number of chiral carbons in the substance's structural formula.
Follow two examples of application of the Vant't Hoff formula:
5-Dichloro-2,3-dihydroxy-hexanedioic acid

In the compound's structural formula, there are three chiral carbons or asymmetric, therefore:
IOA = 2no
IOA = 23
IOA = 8
2,3,4,5-tetrahydroxyhexanal

In the compound's structural formula, there are four chiral carbons (red arrows) or asymmetric, therefore:
IOA = 2no
IOA = 24
IOA = 16
Observation: There is a possibility that a structural formula has two asymmetric carbons that have exactly the same ligands. If this occurs, we consider in the calculations only 1 for the value of n, not 2. See an example:
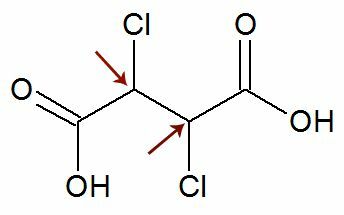
Like two chiral carbons they are equals, we consider only one carbon in the calculation:
IOA = 2no
IOA = 21
IOA = 2
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/isomeros-ativos.htm
