Since there are exceptions to the octet rule, how do we know the correct arrangement between atoms in forming a molecule?
This can be done by calculating the formal load of each structure. The formal charge that is closest to zero will be the one with the greatest probability of actual existence. Note that it's “closest to zero”, so it doesn't have to be zero.
The formal charge formula (Cfo) é:
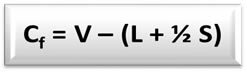
V = number of valence electrons of the free atom;
L = number of electrons present in the isolated (non-bonding) pairs of the atom in the structure;
S = number of electrons shared by the atom in the structure.
To understand how this happens, imagine that we want to know what the Lewis electronic structure is for the SO molecule.2. We have two possible arrangements between atoms:
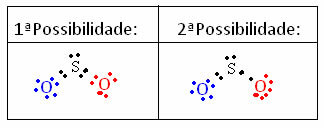
The formal charge of all atoms participating in the structures is calculated. Look:
1st Possibility:
Sulfur(S): Oxygen (O) Oxygen (O)
Çf(S) = 6 – (2 + ½ 8) Çf(S) = 6 – (4 + ½ 4) Çf(S) = 6 – (4 + ½ 4)
Çf(S) =0 Çf(S) =0 Çf(S) =0
2nd Possibility:
Sulfur(S): Oxygen (O) Oxygen (O)
Çf(S) = 6 – (2 + ½ 6) Çf(S) = 6 – (6 + ½ 2) Çf(S) = 6 – (4 + ½ 4)
Çf(S) = +1Çf(S) = -1 Çf(S) =0
Based on the results obtained, we can note that the 1st structure is the one with the highest probability of real existence. So, we know that it doesn't follow the octet rule, but that sulfur has expanded its valence shell, remaining stable with 10 electrons.
This rule also applies to finding the correct ion arrangement.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/calculo-carga-formal.htm
