we have a mixing of solutions with different solutes without chemical reaction when two or more mixtures that have substances with the same ion in common (either the same cation or the same anion). As in the example below:
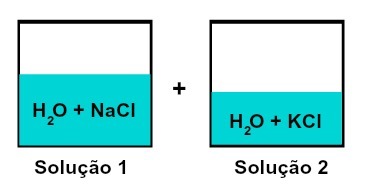
Mixture of solutions that have different solutes
Solution 1 is water and sodium chloride (NaCl), while solution 2 has water and potassium chloride (KCl). When mixed together we have a mixing of different solute solutions without chemical reaction, because both salts used have the chloride anion (Cl-).
1- Characteristics of mixtures of different solute solutions without chemical reaction
When a mixture of solutions that have different solutes without chemical reaction is carried out, the characteristics below are always checked:
The mass of each of the solutes does not change (if in solution 1 we have 10 g of solute and in 2, 30 g, for example, after mixing we will have the same mass of each solute),
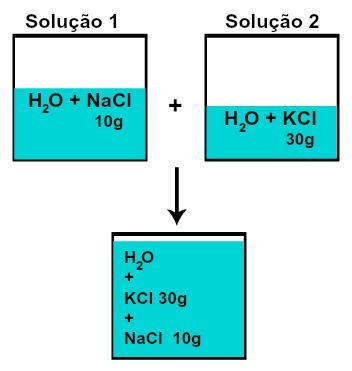
Mass of each of the solutes after mixing solutions without chemical reaction
THE amount of matter (n) of each of the solutes does not change (if in solution 1 we have 5 mol of solute and in 2, 4 mol, for example, after mixing we will have the same amount of matter of each),
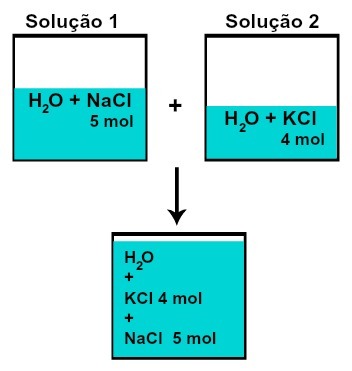
Number of moles of each of the solutes after mixing solutions without chemical reaction
The volume of the final solution, VF, is the result of the sum of the volumes of each of the mixed solutions (if in solution 1 we have 200 mL and in solution 2, 300 mL, for example, after mixing we will have 500 mL of volume),
VF = V1 + V2
2- Formulas used in calculations of mixing solutions of different solutes without chemical reaction.
As in this type of mixture we only have an increase in the amount of solvent in relation to each of the solutes, we must calculate the final concentration of each of the solutes using the following expressions:
a) To common concentration (Ç)
For solution 1: the multiplication of the concentration of solution 1 by its volume is equal to the final concentration multiplied by its volume
Ç1.V1 = CF.VF
For solution 2: the multiplication of the concentration of solution 2 by its volume is equal to the final concentration multiplied by its volume
Ç2.V2 = CF.VF
b) To concentration in quantity of matter or molarity (M)
For solution 1:
M1.V1 = MF.VF
For solution 2:
M2.V2 = MF.VF
c) Concentration in quantity of matter of each ion present in the solution
If we have to determine the concentration of one or all of the ions present in the final solution, we must:
1º: Remember that the ion concentration is given by the multiplication of the concentration (M), of the solute it comes from, by its index in the substance formula. So, for the ion Y, in substance 1, XY3, the concentration will be:
[Y]1 = 3. M
As for solute2, ZY, the concentration of Y would be given by:
[Y]2 = 1. M
2º: If we have more than one solute that releases the same ion, for example, the XY solutes3 and ZY, which have the same ion Y, the concentration of this ion in the final solution is given by the sum of its concentrations for each solute:
[Y]F = [Y]1 + [Y]2
3- Examples of calculations involving mixing solutions of different solutes without chemical reaction
Example 1: (PUC SP) In a beaker, 200 mL of an aqueous solution of calcium chloride (CaCl) were mixed2) of 0.5 mol concentration. L–1 and 300 ml of a 0.8 mol solution. L–1 of sodium chloride (NaCl). The solution obtained has a chloride anion concentration of approximately:
a) 0.34 mol. L–1
b) 0.65 mol. L–1
c) 0.68 mol. L–1
d) 0.88 mol. L–1
e) 1.3 mol. L–1
The data provided by the exercise were:
Solution 1:
Volume (V1): 200 ml
Molar concentration (M1): 0.5 mol. L–1
Solution 2:
Volume (V2): 300 ml
Molar concentration (M2): 0.8 mol. L–1
To determine the concentration of chloride anions (Cl-), we must follow these steps:
Step 1: calculate the volume of the final solution
VF = V1 + V2
VF = 200 + 300
VF = 500 ml
Step 2: Calculate the molar concentration of the final solution with respect to the CaCl solute2, using the expression below:
M1.V1 = MF.VF
0.5,200 = MF.500
100 = MF.500
100 = MF
500
MF = 0.2 mol. L–1
Step 3: Calculate the molar concentration of chloride[Cl-]1, in the final solution, from the CaCl solute2, using the expression below:
NOTE: In the formula we have the multiplication of molarity by 2 because we have index 2 in Cl, in the solute formula CaCl2.
[Cl-]1 = 2.MF
[Cl-]1 = 2. 0,2
[Cl-]1 = 0.4 mol. L–1
Step 4: Calculate the molar concentration of the final solution with respect to the NaCl solute, using the expression below:
M2.V2 = MF.VF
0.8,300 = MF.500
240 = MF.500
240 = MF
500
MF = 0.48 mol. L–1
Step 5: Calculate the molar concentration of chloride, [Cl-]2, in the final solution, from the NaCl solute, using the expression below:
NOTE: In the formula we have the multiplication of molarity by 1 by the fact that we have index 1 in Cl, in the formula for the solute NaCl.
[Cl-]2 = 1.MF
[Cl-]2 = 1. 0,48
[Cl-]2 = 0.48 mol. L–1
Step 6: Calculate the total amount of chloride ions in the final solution
To do this, just add the molar concentrations of chlorides for each of the solutes in steps 3 and 5:
[Cl-]F = [Cl-]1+ [Cl-]2
[Cl-]F = 0,4 + 0,48
[Cl-]F = 0.88 mol. L–1
Example 2: To a solution of 500 ml of 6 mol/L KOH was added 300 ml of K solution.2ONLY3 3 mol/L. What is the concentration of each of the solutes in the resulting mixture
a) 3.75 and 3.0 mol/L
b) 3.75 and 1.215 mol/L
c) 4.5 and 1.125 mol/L
d) 3.75 and 1.125 mol/L
e) 4.5 and 1.215 mol/L
The data provided by the exercise were:
Solution 1:
Volume (V1): 500 ml
Molar concentration (M1): 6 mol. L–1
Solution 2:
Volume (V2): 300 ml
Molar concentration (M2): 3 mol. L–1
To determine the concentration of chloride anions (Cl-), we must follow these steps:
Step 1: calculate the volume of the final solution
VF = V1 + V2
VF = 500 + 300
VF = 800 ml
Step 2: Calculate the molar concentration of the final solution with respect to the KOH solute, using the expression below:
M1.V1 = MF.VF
6,500 = MF.800
3000 = MF.800
3000 = MF
800
MF = 3.75 mol. L–1
Step 3: Calculate the molar concentration of the final solution in relation to solute K2ONLY3, using the expression below:
M2.V2 = MF.VF
3,300 = MF.800
900 = MF.800
900 = MF
800
MF = 1.125 mol. L–1
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/mistura-solucoes-com-solutos-diferentes-sem-reacao-quimica.htm