Condensation polymers, also called elimination polymers, are those in which their monomers are the same or different. unite with the simultaneous elimination of water molecules or other small molecules of compounds that will not be part of the polymer.
The only exception is polyurethane: in the condensation reaction, through which it is obtained, there is no release of molecules.
The main compounds released in addition to water are: hydrogen chloride (HCl), ammonia (NH3) and hydrogen cyanide (HCN).
The condensation polymers will always have a regular, uniform structure, that is, the polymers will always come alternately and not randomly. Copolymers (whose structure is irregular) can only be formed when more than two monomers come together to form the condensation polymer.
Considering water as the molecule that is eliminated, we have the following scheme of the generic condensation reaction for the formation of these polymers:
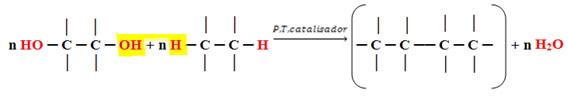
Generic reaction for the formation of condensation polymers.
The most important and most used condensation polymers in our society are:
• Polyurethane: obtained by the condensation of paraphenylene diisocyanate with 1,2-ethanediol. It is used in insulation, rocket fuel binders, clothing linings, upholstery foams, surfboards, etc.;

Products made from polyurethane.
• Bakelite: the substances that give rise to bakelite are benzene and methanol. It is used in coatings such as paints and varnishes, wood glues, pot handles, light switches, sockets, plugs, covers, etc.;
• Polyester: they are polymers formed by several esters, and an acid and an alcohol are needed to form them. The main polyester is PET (polyethylene tereflate), formed by the union of terephthalic acid with ethanediol. It is used in the production of textile fibers, such as tergal fabric, in the production of soft drink bottles and other beverages, video tapes, heart vessels and valves, as a protector to facilitate the recovery of organic tissues that have suffered burns, among other uses;
• Nylon or Polyamide: common nylon monomers (nylon 66) are hexanedioic acid and 1,6-hexanediamine. Its applications can be seen in lubrication-free bearings, gears, packaging, textile fibers, velcros, brush bristles, fishing wires and electrical accessories;

socks made of nylon
• Kevlar®: it is formed by the union between terephthalic acid and p-benzenediamine. It is mainly applied in bulletproof vests as well as in racing car chassis, in race car drivers' clothing, in firefighting clothing and in aircraft parts;

Bulletproof vests for the protection of soldiers and police are made with Kevlar® polymer
• Polycarbonate: Formed by phosgene and p-isopropylenediphenol, polycarbonate is widely used in bulletproof glass, in sunglasses lenses, CDs and DVDs, X-ray equipment, security windows and structures to cover certain areas (like the one shown in the figure bellow);

Structure made on the basis of polycarbonate.
• Silicones: formed by silicon as the main element, where its atoms are alternated with those of the element oxygen and silicon binds to organic radicals. The most common silicone is dichlo-dimethyl-silane. The applications of these compounds are: prostheses placed through plastic surgery, lubrication of molds, window seals, encapsulated resins, cosmetics such as oils and skin creams, among others.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/polimeros-condensacao.htm
