Substitution reactions, in general, are those in which at least one hydrogen atom of a hydrocarbon molecule is replaced by a substituent group.
1- Nitration: Reaction between nitric acid (HNO3) and an organic compound.
Example: Reaction between Benzene and nitric acid.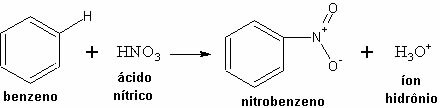
Benzene can also react with nitric acid in the presence of sulfuric acid (H2ONLY4) which, being a stronger acid than HNO3, makes it behave like a Lewis base, receiving a proton from the H2ONLY4. See the equation that represents the process: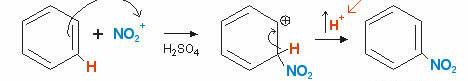
In this Nitration reaction, an acid-base balance occurs.
2- Sulphonation: this reaction has sulfuric acid as reactant (H2ONLY4).
Example: Sulphonation of the benzene ring.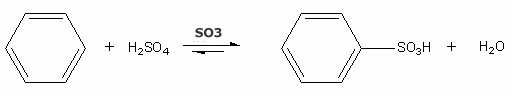
Note that the reaction takes place in the presence of a catalyst - sulfur trioxide (SO3), and is carried out under heating. The products of the reaction are benzenesulfonic acid and water.
3- Halogenation: Even from the name, it can be deduced that in Halogenation, the hydrogen atom is replaced by halogen atoms (F, Cl, Br, I). The most common are chlorination and bromination.
Example: Propane Monobromination
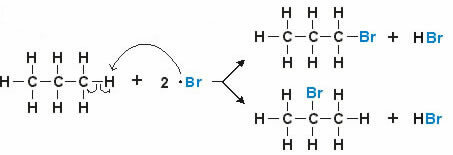
The ligand H of the propane molecule is replaced by the ligand Br, giving rise to 1 -Bromine propane or 2 -Bromine propane.
Note: The two arrows indicate that the halogen (Bromine) can replace any hydrogen in the structure.
Example: Chlorination of benzene.
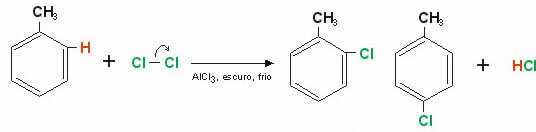
Note that one of the hydrogen atoms present on the benzene ring has been replaced by an atom of Chlorine, this can occupy both the ortho and the para positions, hence the products: o-chlorobenzene or p-chlorobenzene.
By Líria Alves
Graduated in Chemistry
Brazil School Team
See more!
Hydrocarbon branches
Organic chemistry - Chemistry - Brazil School
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacoes-substituicao-hidrocarbonetos.htm


