Common concentration (C), or simply concentration, is defined as: "the ratio between the mass of the solute and the volume of the solution." Mathematically, it is expressed by the formula:

C = Common concentration, whose unit in the System Iinternational of Mmeasures (SI) is given in g/L;
m1 = mass of solute* in g;
v = volume of solution in L.
Particularities and measurement units of common concentration
The symbol C in the above formula is common, not concentration, as there are other types of concentration, such as molar concentration or molarity, concentration on parts per million or ppm, percentage by mass of the solute or mass title, volume concentration etc.
As shown above, the concentration of a solution has a standard unit of g/L (grams per liter), but it can be expressed in other units of mass and volume, such as g/m3, mg/L, kg/mL, etc.
If we say that a solution of water and sugar has a concentration of 50 g/L, it means that in each liter of the mixture (solution) there is a dissolved mass of 50 g of sugar.
Example of application of common concentration in everyday life
In everyday life, the concentration it is widely used to indicate the composition of foods, medicines and liquid cleaning and hygiene materials. Look at the label of whole milk below where the concentration of various nutrients is listed, such as carbohydrates, proteins and total fats present in 200 ml of the solution.

For example, in every 200 ml of milk, there are 9 g of carbohydrates. From the transformation of this amount of milk to liters and the calculations as shown below, the concentration existing carbohydrates is 45 g/L.
Transformation of the volume unit for the International System, that is, from mL to L:
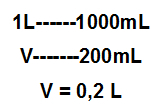
Calculation of concentration of carbohydrates in milk:

This means that for every liter of this milk, 45 g of carbohydrates are found.
Note: In quantitative aspects related to chemical solutions, whenever the index 1 appears in a quantity, it indicates that the quantity is only for the solute. The index 2 refers to the solvent and, when there is no index, it is referring to the entire solution (solute + solvent).
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/concentracao-comum-c.htm


