THE Chemical Kinetics is a field that studies the factors that influence the rate of development of chemical reactions, that is, the speed at which they take place. There is more and more research around this area, as we often want to speed up some reactions or slow down others. This is especially important for industries and this research has important technological consequences.
But since the speed of a reaction can vary at each time interval and from one substance to another, it is customary to calculate the average reaction speed.
Consider the following generic reaction, where coefficients are lowercase letters and reactants and products are represented by uppercase letters:
a A + b B → c C + d D
The average speed of this reaction will be given dividing the average rate of reaction of any one of the reacting substances or the average rate of formation of any one of the products by its respective coefficient in the chemical equation. This is given by:
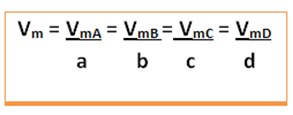
For example, consider the hydrogen peroxide decomposition reaction:
2 hours2O2(aq) → 2 H2O(1) + 1 O2 (g)
Let's say that in 1 minute 0.3 mol/L of H was formed2O and 0.15 mol/L of O2, while 0.3 mol/L of H decomposed2O2, that is, the average rates of decomposition and formation of these substances in the reaction are:
VmH2O2 = 0.3 mol/L. min
VmH2O = 0.3 mol/L. min
VmO2 = 0.15 mol/L. min
The average speed of the global reaction will be found by taking one of these values and dividing it by the respective coefficient in the equation:
Vm = VmH2O2 = 0.3 mol/L. min = 0.15 mol/L. min
2 2
Vm = VmH2O = 0.3 mol/L. min = 0.15 mol/L. min
2 2
Vm = VmO2 = 0.15 mol/L. min = 0.15 mol/L. min
1 1
Note that the three values are equal, therefore, the reaction rate is the same as a function of any reactant or product, in the same time interval.
However, how were the values of the average velocities of each of the substances involved in these reactions found?
It can be calculated dividing the variation in the concentration of the substance (reagent or product) by the time interval. If we are going to determine the average speed of one of the reagents of the reaction, we will have to put a negative sign before, or else consider the concentration value in module ||, since, since the concentration of the reactant decreases over time, the velocity value would be negative, but there is no negative velocity.

For example, consider the following ozone gas decomposition reaction (O3(g)) in oxygen gas (O2(g)):
2 O3(g) → 3 O2(g)
Let's say that in a balloon there was 10 moles of ozone gas, but after 1 minute, only 4 moles were left; this means that 6 moles of ozone turned into oxygen gas. So we have:
2 O3(g) → 3 O2(g)
t = 0 min 2 mol/L 0 mol/L
expenses formed
6 mol/L 9 mol/L
t = 1 min 4 mol/L 9 mol/L
Note that since the reaction ratio is 2:3, then if 6 moles of O were used3, 9 mol of O were formed2. So, after 1 minute, we have the following average speeds:
Vm = - ∆ [The3]
t
Vm = - ([O3final - O3initial])
tFinal - tinitial
Vm = - ([4 - 10])
1– 0
Vm = 6 mol/L. min→ For 1 minute, 6 mol of ozone reacted in each liter of the system.
Vm = ∆ [The2]
t
Vm = ([O2final - O2initial])
tFinal - tinitial
Vm = ([9 - 0])
1– 0
Vm = 9 mol/L. min→ During 1 minute, 9 moles of oxygen were formed in each liter of the system.
This shows us that we can calculate the average velocity as a function of the reactants or as a function of the products.
Now, if we want to calculate the average speed of this global reaction, just do as we showed at the beginning: divide each of these speeds by their respective coefficients in the chemical equation:
Vm = VmO3 = 6 mol/L. min = 3 mol/L. min
2 21
Vm = VmO2 = 9 mol/L. min = 3 mol/L. min
3 3
Vm = VmO3 = VmO2
23
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/velocidade-das-reacoes-quimicas.htm