Lorenzo Romano Amedeo Carlo Avogadro (1776-1856) was an Italian chemist who first established the idea that a sample of an element, with mass in grams numerically equal to its atomic mass, always has the same number of atoms (N).
Avogadro himself was unable to determine the value of N. However, throughout the twentieth century, the advancement of technology and scientific knowledge made it possible for other scientists to develop techniques to determine it. When this value was finally discovered, it was called Avogadro's constant, in honor of this scientist, as it was he who laid the foundations for its creation.

Lorenzo Romano Amedeo Carlo Avogadro (1776-1856)
In 1 mole of any entity (atoms, molecules, electrons, formulas or ions) is contained exactly the value of Avogadro's constant.
The table below shows some values for Avogadro's constant obtained throughout the 20th century:
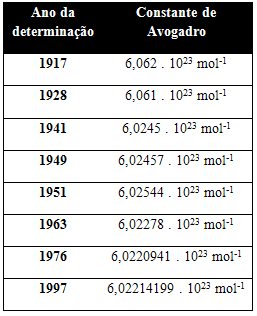
Here are some of the techniques used to try to determine the value of Avogadro's constant:
The first scientist to make an approximate calculation for Avogadro's constant was Johann Joseph Loschmidt. In the year 1867, he based himself on the kinetic theory of gases and determined how many molecules existed in 1 cm
3 of a gas.Another of these scientists was the Frenchman Jean Baptiste Perrin (1870-1942) who counted the number of colloidal particles per unit volume in a suspension and measured their masses. The value he found was between 6.5 and 7.2. 1023 entities per mol. This scientist published, in 1913, the book Les Atomes (1st ed. Paris: Alcan), and its 9th edition, published in 1924, contained 16 ways to experimentally obtain Avogadro's constant.

Jean Baptiste Perrin (1870-1942)
Years later, scientist James Dewar (1842-1923) used a method developed years earlier by radiochemist Bertram Boltwood (1870-1927) and physicist Ernest Rutherford (1871-1937), which basically consisted of counting the alpha particles emitted by a radioactive source and determining the volume of helium gas obtained. The value found by Dewar was 6.04. 1023 mol-1.
Back in the 20th century, Robert Millikan (1868-1953) carried out an experiment to determine the charge of the electron (1.6. 10-19 Ç). As the charge of 1 mole of electrons was already known (96500 C), it was possible to relate these two values and find the following value for Avogadro's constant: 6.03. 1023 mol-1.
Currently, the recommended value for Avogadro's constant is 6.02214 x 1023 mol-1 and it is determined by means of X-ray diffraction, in which the volume of a few atoms of a crystalline lattice is obtained, as long as the density and mass of 1 mole of atoms in the sample is known.
For didactic purposes, in High School, where calculations do not need to be as accurate as those performed in chemical laboratories, Avogadro's constant is considered as 6,02. 1023 mol-1.
There are also simpler methods that can be used to help students determine Avogadro's constant in practice. One of them is through electrolysis in an aqueous medium.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/determinacao-constante-avogadro.htm
