O luminol is a powdered organic compound whose molecular formula is C8H7O2N3. This chemical reagent is used by mixing it with hydrogen peroxide (H2O2, its solution is called hydrogen peroxide), some hydroxide and other chemicals, which give rise to a liquid solution.
When luminol and hydrogen peroxide react, they emit a blue fluorescent light. However, as this reaction is very slow, it is not possible to observe it with just a mixture of the two. In contact with blood, on the other hand, the iron in hemoglobin acts as a catalyst and accelerates this reaction, with only five seconds for radiant light to appear. Therefore, when the luminol solution is sprinkled on a bloody spot, a chemiluminescence is observed.
See the chemical structure of luminol:
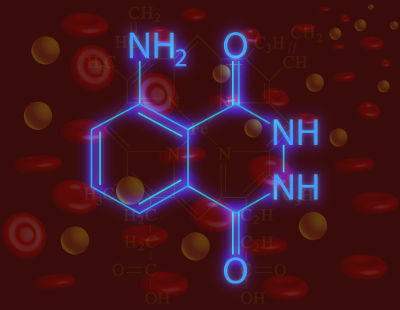
Structural formula of pure luminol
Luminol is widely used in forensics to identify blood at the scene of a crime. Even if the place or other items, such as clothing, carpets, etc., have been washed before, the fibers of the fabric absorb parts of the iron compound and yet the place is “illuminated” by the use of the luminol. With the dark place, you can clearly see the blue dots that indicate the presence of blood.
The term “chemiluminescence” is used to refer to a chemical reaction that generates light. And that's exactly what happens in the oxidation reaction between luminol and hydrogen peroxide. Luminol loses nitrogen and hydrogen atoms and gains oxygen atoms, forming 3-aminophthalate. The electrons in this compound are at a higher energy level than the reactants and, being an unstable state, the electrons return to an energy state lower, emitting the extra energy in the form of visible electromagnetic waves, that is, in the form of light, which we see in the color wavelength range blue.
Luminol is typically sprayed at a crime scene only after experts have considered other options, as it can destroy other evidence at the crime scene. In addition, one aspect that must be taken into account is that it is not only the blood that makes luminol “glow”, but other compounds as well, such as bleach. Therefore, investigators need to perform further tests to verify that it really is human blood.
Luminol cannot be sprayed on metallic surfaces either, as, as explained above, it is the iron that catalyzes the reaction that makes the blue color appear. So iron from these surfaces could give a false positive result.
* Image credit: Author: everyone's idle /Fonte: Wikimedia Commons.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-luminol.htm

