Dry ice is so called because despite having the appearance of ice (water in the solid state), it is actually solid carbon dioxide (carbon dioxide - CO2). And, unlike ordinary ice that melts, it passes directly into a gaseous state, that is, it sublimes.
This is the most important property of dry ice, because when it changes to a gaseous state, it drags water molecules with it, creating a mist denser than air. thanks to that "White smoke" formed, the dry ice it is widely used as a scenic resource in films, concerts, theaters, television programs and parties.
But, the question arises: what is different about dry ice from other substances involved in its sublimation? What happens between your molecules?
Carbon dioxide is non-polar, so when it's in a solid state, like dry ice, its molecules stay in molecular crystals thanks to an intermolecular force of attraction between them, which is the induced dipole. This force arises when your molecules get closer and there is a repulsion between your electrons, leading to momentary deformations in your electronic clouds. This means that momentary dipoles appear in the molecules, which induce adjacent molecules, resulting in attractive forces.
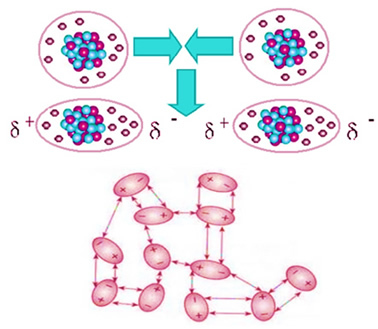
This is the only intermolecular force present in the nonpolar molecules of dry ice and its intensity is very small. The induced dipole force is the weakest of all. Thus, it takes little energy to break these forces of attraction and make the compound change its physical state. That's why dry ice sublimes at -78,6°C, under a pressure of 1 atm.
Other examples of substances that also have this type of intermolecular interaction and that also sublimate are mothballs it's the solid iodine.

By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/por-que-gelo-seco-sublima.htm

