Covalent bonding occurs when two atoms share pairs of electrons in order to acquire the electron configuration of an noble gas (with 8 electrons in the valence shell or with 2, in the case of those with only the K shell), according to the rule of octet.
However, there are special cases of covalent bonds in which the shared electron pair comes only from one of the atoms that is already stable. Previously, this type of covalent bond was called dative, today it is more commonly called coordinate.
See some examples to understand how this happens:
- CO (carbon monoxide):
Carbon has 4 electrons in its valence shell. So, according to the octet rule, to be stable, it needs to receive 4 more electrons, totaling 8. Oxygen, on the other hand, has 6 electrons in the valence shell and needs to receive 2 electrons to get the configuration of the noble gas neon.
So, first, carbon and oxygen share two pairs of electrons so that oxygen is stable:
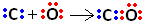
However, carbon remains unstable as it still has 6 electrons and needs 2. Therefore, oxygen, which is already stable, shares one of its pairs of electrons with carbon, that is, it makes a dative bond with it, making it stable:

Note that the coordinate covalent bond can be represented by a dash, just like the common covalent bond.
- ONLY2 (Sulfur dioxide):
Each sulfur and oxygen atom has 6 electrons in their valence shells, so they need to receive 2 electrons each. Initially, sulfur makes two common covalent bonds, sharing two pairs of electrons with one of the oxygen atoms, both remaining stable with 8 electrons.
But the other oxygen atom is not stable, so sulfur shares a pair of its electrons with it via a coordinated or dative covalent bond:
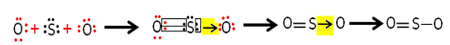
Note that in this case we have three atoms in the molecule, so there can be a migration of the bond from one atom to another. The sulfur dioxide molecule can also be represented like this: O ─ S ═ O.
We call this bonding phenomenon resonance. See three more examples in the table below:
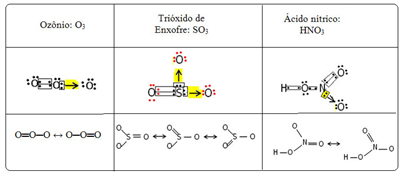
Possible structures are called resonance structures or canonical forms.
This type of bond also occurs in the formation of ions, as in the case of hydronium ions (H3O+) and ammonium (NH4+).
First, the H cation+ it forms when hydrogen loses its single electron, leaving it with a positive charge. So it will need to receive two electrons to be stable. This happens through a dative bond with water (in the case of the hydronium ion) and with ammonia (in the case of the ammonium ion). Watch:

By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/ligacao-covalente-dativa-ou-coordenada.htm
