To the nitrogen functions are a group of organic compounds that have atoms of nitrogen attached to the carbon chain. They are distinguished by their respective functional groups, which are sets of atoms connected in a certain configuration and which define the characteristic properties of each function.
The nitrogen functions are:
amines;
amides;
nitro compounds;
nitriles;
isonitriles.
Read too:What are the oxygen functions?
Summary of nitrogen functions
Nitrogen functions are organic compounds that contain atoms of nitrogen.
Amines, amides, nitrocompounds, nitriles and isonitriles are the nitrogen functions.
Inorganic functions are distinguished by their functional group.
Amines are derived from the substitution of hydrogens of ammonia by alkyl radicals.
Amides have the same carbon atom joined to nitrogen and carbon. oxygen.
Nitro compounds contain the -NO group.2.
Nitriles are characterized by the presence of a triple bond between carbon and nitrogen.
Isonitriles have a triple bond between nitrogen and carbon, with nitrogen attached to the carbon chain.
Video lesson on nitrogenous functions
What are nitrogen functions?
Nitrogen functions are a set of organic functions that have the nitrogen atom in their structure, in addition to carbon and hydrogen atoms. They are: amines, amides, nitrocompounds, nitriles and isonitriles,
What differentiates the nitrogenous functions are the respective functional groups, which are the structural arrangement of atoms responsible for the properties of the substance.
What are the nitrogen functions?
amines
The organic function the mine is characterized by bonding at least one carbon chain to the nitrogen atom. The functional group of amines is -N-R1R2R3 (the R groups are carbon chains or hydrogen atoms, at least one of which is a carbon chain).

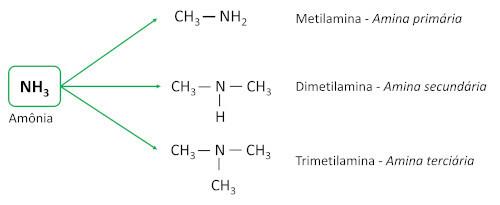
the amines are derived from ammonia (NH3), with one or more hydrogen atoms replaced by carbon atoms or alkyl ligands. They are basic substances, leaving the pH greater than 7 in aqueous solution.
They are classified according to the amount of hydrogen atoms replaced by carbon ligands (alkyl or aryl groups), which are called substituents.
- Primary amines: have a single carbon substituent.
- Secondary amines: have two carbon substituents.
- Tertiary amines: have three carbonic substituents, that is, the nitrogen atom is no longer bonded to any hydrogen atom, establishing three Connections simple with carbon atoms.
A nomenclature for amines follows the guidelines of IUPAC (International Union of Pure and Applied Chemistry). In the case of primary amines, the construction of the name takes into account the number of carbons (prefix), the nature of the bond between carbons (infix) and the term is used the mine for the suffix (end of word).

See the example:
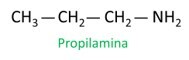
When assigning numbers to carbons for the location of radicals, assign the lowest possible number to the atom closest to the NH group.2.
For secondary and tertiary amines, the nomenclature considers the longest substituent group attached to the nitrogen as the main chain, and the other ligands are written with the prefix referring to the number of carbons and the suffix -il, with the letter N before them.

Example:
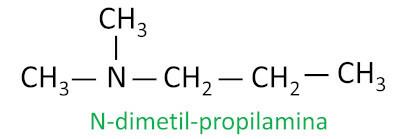
Amides
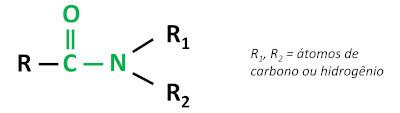
The organic function amide is characterized by bonding a carbonyl group (C=O) to the nitrogen atom, where nitrogen can be bonded to carbon or hydrogen atoms.
The amides are basic substances, leaving the pH above 7 in aqueous solution. The substance urea belongs to the group of amides and is a compound naturally present in urine, derived from degradation processes that occur in living organisms.
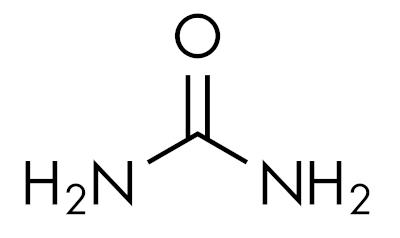
Amides are characteristic depending on the level of nitrogen substitution. Like this:
- Primary amides: hold two hydrogen atoms together with nitrogen.
- Secondary amidesor monosubstituted: one of the hydrogens has been replaced by carbon chain, so nitrogen maintains a single bond with hydrogen.
- tertiary amidesor displaced: Nitrogen no longer has hydrogen bonds, all of which have been replaced by carbons.

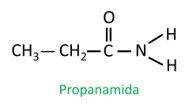
Amides are Named according to IUPAC, the molecules being named by the sequence:

In branched amide, the carbon atom of the amide group participates in the main chain, and the carbon count must start with it. Look:
 |
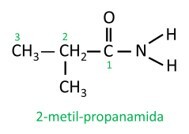 |
In monosubstituted or disubstituted amides, the letter N indicates the position of the branch which is attached to the nitrogen atom. If there is another radical in the chain, it will be written after identifying the radical referring to N.
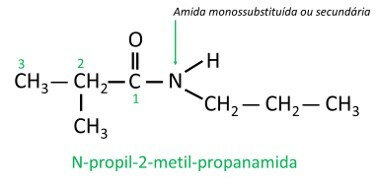
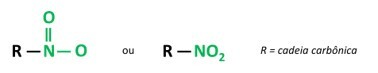
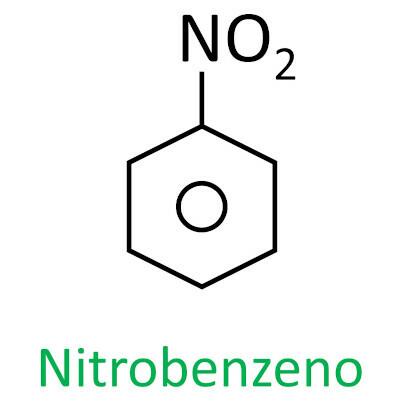
nitro compounds
You nitro compounds are organic compounds that contain a nitro group (-NO2) attached to a carbon chain, which can be aliphatic (open or linear) or aromatic.
A main characteristic of these substances is their explosive power. The explosive capacity is associated with aromatic nitrocompounds, and the greater the number of nitro groups, the greater the explosion. Aliphatic nitro compounds are used in laboratories as organic solvents.
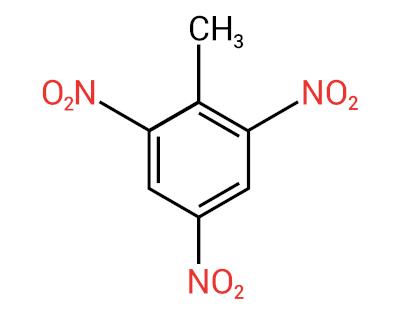
The nomenclature for these compounds is formed by the word nitro followed by the name of hydrocarbon (prefix + infix + ending -O).
See some examples:
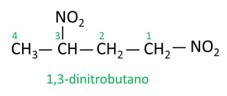 |
 |
nitriles
Nitriles are organic compounds characterized by the triple bond between a carbon atom and a nitrogen atom, presenting the functional group – C ≡ N. In nitriles, the nitrogen is at the end and the carbon is attached directly to the carbon chain.

the nitriles Also known as cyanides, as they derive from reactions with hydrocyanic acid (HCN).
they are substances toxic to humans, because within the body they can form hydrocyanic acid in contact with stomach acid, preventing processes of cellular respiration of the cells.
In nature, nitriles can be found in some stone fruits, but in a very low concentration, not presenting risks, and in the leaves of wild cassava.

The Iupac rule for the nomenclature of nitriles defines that the term nitrile be added as a suffix:

See some examples:
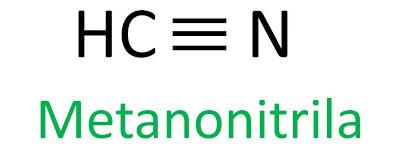 |
 |
The usual form of nomenclature for nitriles is cyanide + radical name.
isonitriles
Isonitriles, or isonitriles, are compounds formed by the triple bond between a carbon atom and a nitrogen atom, presenting the functional group – R ≡ C. In isonitriles, the carbon is at the end and the nitrogen is attached directly to the carbon chain.

The isonitriles differ from the nitriles due to the position of the nitrogen and carbon atoms, and In isonitriles, nitrogen is characterized as a heteroatom., as it is positioned between two carbons.
They are unstable species and can convert to nitriles at elevated temperatures. Isocyanidic acid reactions give rise to isonitriles, which is why these compounds are also known as isocyanides.
Isonitrile molecules follow the Iupac rule for naming:
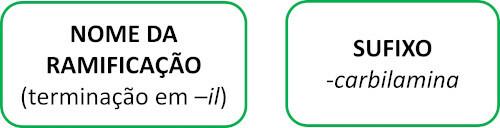
See some examples:
 |
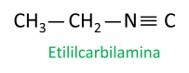 |
Applications of nitrogen functions
The substances belonging to the nitrogenous functions have numerous applications, mainly in the industrial area, for the manufacture of polymers, rubber, synthetic fibers, pharmaceuticals, agrochemicals, pesticides and explosives.
To the amines are used in the production of dyes, soaps, drugs, in the process of rubber vulcanization, explosives and other industrial processes, and are also found in plants and formed in organic matter decomposition processes.

To the amides have wide application in the industrial and chemical sector, being present in the production of polymers (such as nylon and polyurethane), resins, explosives, fertilizers, insect repellents, fertilizers and drugs.

You nitro compoundsIts main application is the manufacture of explosives. that are used in the military, industrial and metallurgical sector (for raw material extraction). One of the best known nitrocompounds is trinitrotoluene, popularly known as TNT. Other applications of nitrocompounds are in the production of pesticides, bactericides, dyes, petroleum refining, etc.

To the nitriles are used as organic solvents in the laboratory and in industry, participating in the extraction and manufacturing processes of some synthetic fibers, plastic polymers, dyes and fertilizers.

To the isonitriles are employed in various processes for the production of organic compounds, such as solvents, and participate in the manufacture of agrochemicals, pesticides, rubbers and plastics.
Read too:Halides — substances that have halogen atoms attached to the carbon chain
Solved exercises on nitrogen functions
question 1
(FPS-PE-modified) The application of nitrogenous compounds in synthetic organic chemistry is very diversified and involves the preparation of drugs, dyes, explosives and vitamins. Look at the compounds below.
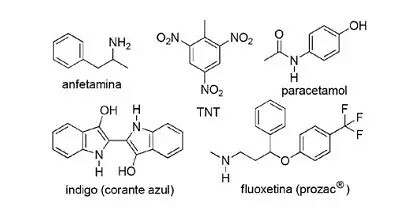
About these compounds, mark the incorrect statement.
a) TNT is a nitro compound.
b) The nitrogenous portion of fluoxetine is a secondary amine.
c) Amphetamine is classified as a primary amide.
d) Indigo has heteroaromatic rings in its structure.
e) The nitrogenous portion of paracetamol is an amide.
Resolution:
Letter C
The item The is correct, because TNT is a nitro compound because it has NO groups2.
The item B is correct. The nitrogenous portion of fluoxetine is a secondary amine because it is linked to two carbon segments.
The item w is incorrect, as amphetamine is classified as a primary amine, not an amide. Note that the present group is NH2. So this is the gist of the question.
The item d is correct, as indigo has heteroaromatic rings in its structure, that is, rings formed by carbon atoms and another element — in this case, nitrogen.
The item It is is correct, because paracetamol is an amide, presenting carbon linked to nitrogen and oxygen.
question 2
(UFMS) Yerba mate (Ilex paraguariensis), originally from South America, is used as a tonic and stimulant drink. The product obtained by processing yerba mate leaves can be used to prepare chimarrão and tereré, among other drinks commonly and culturally consumed in regions of Argentina, Paraguay and Brazil. The great interest in yerba mate is due to the chemical compounds present, due to its antioxidant, stimulant and diuretic properties.
(Available in: http://repositorio.utfpr.edu.br/jspui/bitstream/1/3158/1/PG_PPGEP_Henrique%2C%20 Flavia%20Aparecida_2018.pdf. Accessed on: 01 Nov. 2018. Adapted).
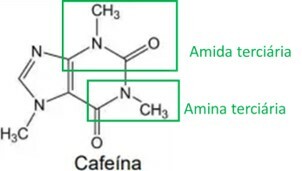
The stimulant properties of yerba mate are related to its methylxanthines content, one of the main ones being caffeine, structure presented below:

When analyzing the structural formula of caffeine, it is correct to state that it has the following organic functions and properties:
a) aldehyde and amide, basic.
b) amine and amide, alkaline.
c) amine and ketone, alkaline.
d) ketone and amide, amphoteric.
e) carboxylic acid and amine, basic.
Resolution:
Letter B
Analyzing the caffeine structure, the amine and amide functional groups are identified, as highlighted in the image below. As these two groups have a basic or alkaline characteristic, caffeine also has this characteristic, meaning that aqueous caffeine solutions have a pH above 7.
By Ana Luiza Lorenzen Lima
Chemistry teacher
Source: Brazil School - https://brasilescola.uol.com.br/quimica/funcoes-nitrogenadas.htm
