Most students know the photosynthesis process performed by plants, algae and certain species of bacteria as the process by which they produce their food (beings autotrophs). However, such students do not really understand how this type of reaction occurs and how it results in plant nutrition. It is necessary to understand this phenomenon of photosynthesis chemically.
The plant removes water and some inorganic molecules (compounds that do not have carbon as the main element of its structure, with some exceptions) from the soil through the root and, together with carbon dioxide (carbon dioxide - CO2) absorbed by plants and in the presence of light, organic molecules are then produced (structures that contain carbon as the main element). An example of an organic molecule produced is glucose (C6H12O6), which, through other transformations, will form starch, cellulose, proteins, amino acids and other constituents of vegetables:
6CO2(g) + 6H2O(1) + sunlight → C6H12O6(aq) + 6O2(g)
As stated, for photosynthesis to occur it is necessary that solar energy is absorbed by the plant. This is done by its pigments, which are substances characterized by emitting a certain color when exposed to light. The main pigment of plants is the
chlorophyll, whose structure is shown below. Its structure is complex, with a Mg ion2+ coordinated in the central cavity, and it is this pigment that is responsible for the green color of plants, because it absorb the wavelengths of red, orange, blue and violet, but reflect much of the light green.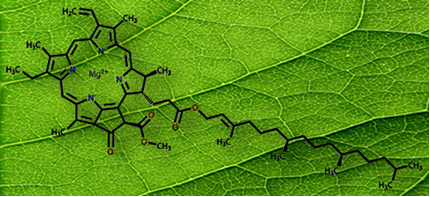
Chlorophyll and other photosynthetic pigments (such as carotenoids and the phycobilins) absorb photons, which causes the electrons in their molecules to become excited, that is, to absorb energy and jump to an orbit farther away from the atomic nucleus, with a higher energy level. These electrons are transmitted to the electron transport chain to be used in the production of ATP (adenosine triphosphate) and then in the synthesis of sugars.
The water molecule is then broken down (oxidation) and the hydrogen supplies electrons to the pigments, in this case to chlorophyll, which has lost its excited electrons. In the breaking of water there will also be the release of O2. In fact, it is interesting to note that practically all the oxygen present in the atmosphere comes from photosynthesis.
The energy obtained is then used to transform (reduce) the CO molecules2 in complex compounds such as carbohydrates and biomass.
Generic photosynthesis reaction:
nCO2 + nH2O+ sunlight →{CH2O}no + nO2
See that this reaction is a reaction of redox, because the oxygen underwent an oxidation, and its Nox (oxidation number – electrical charge of the chemical species) increased, that is, it lost electrons. Hydrogen, on the other hand, reduced, that is, it gained electrons.

From the point of view of chemical reaction, photosynthesis is the opposite of breathing performed by heterotrophic beings (beings, including man, who do not produce their own food, but who need to draw energy from other sources, such as by feeding plants and animals).
In photosynthesis, from light, water and carbon dioxide, organic molecules are synthesized and oxygen is released. In our case, we consume other beings and oxygen to obtain energy for breathing, in which water and carbon dioxide are formed.
Also, when the plant decomposes, it turns into glucose and, over time, the glucose will form CO again.2, in a reaction that is not the inverse reaction of photosynthesis and carbon dioxide will return to the atmosphere.
So we have the carbon cycle.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacao-quimica-envolvida-na-fotossintese.htm
