Matter can undergo two main types of transformations, physical and chemical. Physical transformation is when the nature of matter, that is, its composition, is not changed. For example, when we cut a piece of wood, it underwent a transformation, but it was a physical phenomenon, because it is still wood, its constitution is the same as in the beginning.
On the other hand, a chemical transformation or phenomenon occurs when the nature or composition of matter is changed. In this case, the initial particles (which can be molecules, atoms, ionic clusters, ions, etc.) are like disassembled and their atoms rearrange themselves, assembling new molecules, clusters, atoms, ions etc., that is, new substances. This is a chemical reaction.
For example, imagine that we bring a lighted matchstick close to ethyl alcohol. We know what will happen: the alcohol will start to burn. This means that it is undergoing a chemical reaction with oxygen in the air (O2) and will change its composition, ceasing to be ethanol (C
2H6O), and oxygen will also no longer have its initial composition, originating new substances, which are carbon dioxide (CO2) and water (H2O).
Alcohol on fire - combustion reaction
In chemical reactions, the initial substances are called reagents and the end of products, and the reactions are represented through chemical equations, which follow the following general structure:
REAGENTS → PRODUCTS
Considering the previous example of the complete combustion reaction of alcohol (ethanol), we have the following chemical equation:
Ethanol + Oxygen Gas → Carbon Dioxide + Water
This chemical reaction is represented below by means of atoms according to the Dalton model, in the form of simple spheres:
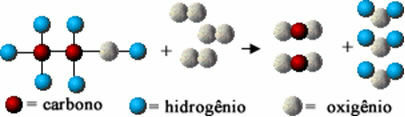
Model representation of an ethanol combustion reaction
Notice that the initial bonds between the atoms were broken and they formed new bonds, and new substances appeared.
Thus, chemical equations are written using formulas and symbols that best represent the above scheme. In the case considered (ethanol combustion reaction), we have that the chemical equation is expressed as follows:
Ç2H5oh(?) + 3 O2(g) → 2 CO2(g) + 3 H2O(v)
There are some visual factors that indicate that there was a chemical reaction, which are:
* Gas release;
* Color change;
* Precipitate formation;
* Appearance of flame or luminosity.
Important processes that occur in our body, in nature and in industries, such as the production of medicines and industrialized foods, are chemical reactions. Therefore, they are extremely important for the emergence and maintenance of life.
There are several types of chemical reactions, which can be classified according to various criteria, but the main types studied in chemistry are:
1. Inorganic reactions: They are usually classified according to the number of substances formed, number of reagents and the presence or absence of simple and compound substances. There are four main types of inorganic reactions:
1.1. Synthesis or addition reactions;
1.2. Decomposition or analysis reaction;
1.3. Simple exchange, displacement or redox reaction;
1.4. Double exchange or metathesis reaction;
2. Organic reactions: These are the ones involving carbon compounds. They are generally classified into three main types:
2.1. Addition Reactions;
2.2. Replacement Reactions;
2.3. Elimination Reactions.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-uma-reacao-quimica.htm


