O neptunium, symbol Np and atomic number 93, is a metal belonging to the actinide series. It is a gray colored metal, but of synthetic origin. Of the 22 existing isotopes of Np, all have a half-life less than the lifetime of Np. planet, and, therefore, it is no longer possible to find appreciable amounts of this element in sources natural.
In the year 1940, neptunium was the first actinide to be synthesized, by means of neutron irradiation techniques in uranium isotopes. Although there are no commercial applications for this element, neptunium can be used in the manufacture of plutonium isotopes, which have specific nuclear applications.
Read too: Complete and updated Periodic Table
Topics of this article
- 1 - Summary about neptunium
- 2 - Properties of neptunium
- 3 - Characteristics of neptunium
- 4 - Where can neptunium be found?
- 5 - Obtaining neptunium
- 6 - Applications of neptunium
- 7 - History of Neptunium
summary about neptunium
Neptunium, symbol Np, it's a metal belonging to the group of actinides.
In metallic form, it has a gray color.
It is reactive with air and dilute acids. There are already several known neptunium compounds.
There are 22 known isotopes of neptunium, with mass 237 having the longest half-life.
It is not possible to find neptunium in appreciable quantities in nature, and therefore it is a synthetic element.
The main form of production is by neutron irradiation to uranium isotopes.
There are no commercial uses for neptunium.
It was discovered in 1940 by McMillan and Abelson.
properties of neptunium
Symbol: No.
atomic number: 93.
atomic mass: 237 a.u.m.a.
electronegativity: 1,36.
Fusion point: 644 °C.
Boiling point: 3902 °C.
Density: 20.25 g.cm-3 (20°C).
electronic configuration: [Rn] 7s2 5f4 6d1.
chemical series: metals, group 3, actinides, internal transition elements.
characteristics of neptunium
Neptunium, symbol Np, is a metal belonging to the actinide series, located in the seventh period, group 3, of the Periodic Table. In its metallic form, neptunium features silver coloring and forms a thin oxide layer when exposed to air at room temperature. At higher temperatures, the oxide formation reaction is more pronounced. In aspects of handling, metallic neptunium resembles uranium.
In aqueous solutions, neptunium admits oxidation numbers between +3 and +7. It reacts with dilute acids and releases hydrogen gas., H2, but is not attacked by bases. Forms tri and tetrahalides, such as NpF3, NpF4, NpCl4, NpBr3 and NpI3, as well as oxides of different compositions, such as Np3O8 and NpO2.
Twenty-two isotopes of Np are known, the 237Np an isotope with a sufficient half-life (2.144 x 106 years) to be handled in measurable quantities.
Do not stop now... There's more after the publicity ;)
Where can neptunium be found?
Neptunium was the first transuranic element to be synthesized, that is, it was lab-produced. Thinking that planet Earth is about 4.5 x 109 years, not even the longest-lived isotope of Np, that of mass 237, would manage to be present in detectable quantity.
Even so, traces of Np can be detected through decay processes of uranium atoms present in mineral samples. However, it is estimated that the amount of Np present is one quadrillionth of the amount of uranium in the ore.
Obtaining Neptunium
The main isotopes of neptunium are produced by neutron irradiation to uranium. Of the 22 known isotopes, only three have half-lives long enough to accumulate: those of mass 235, 236, and 237. The synthesis of 237Np is as follows.
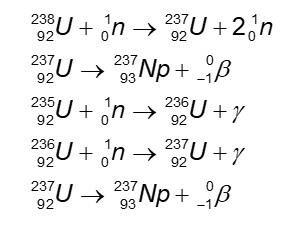
Isotopes 238 and 239 are also produced with the 237Np, however, have a very short half-life and do not accumulate. Isotopes 235 and 236 are synthesized by irradiation of 235U on the cyclotron.
Read too: Actinium — another rare and hard-to-obtain metal
applications of neptunium
There are no commercial uses for neptunium. However, the 237Np is used for the synthesis of 238Pu (plutonium-238). Plutonium, in turn, is used as a heat source for radioisotope thermoelectric generators and radioisotope heat units. The first is used to provide electricity for space vehicles on NASA missions such as Galileo, Cassini and Ulysses. The second is used to provide heat for delicate instruments in space missions.
Metallic neptunium can be obtained by reducing NpF3 with barium or lithium vapors at a temperature of approximately 1200 °C.
history of neptunium
Neptunium was the first actinide to be synthesized in the laboratory. In the year 1940, McMillan and Abelson bombed a thin layer of uranium oxide VI (UO3) with neutrons in a cyclotron. The results pointed to two new radioactive components being created: one with half life time of 23 minutes (later identified as 239U) and another with a half-life of 2.3 days.
After extensive investigation of the results, it was concluded that the other component, with a longer half-life, was the element with atomic number 93, with mass equal to 239.
The new element was called neptunium (the spelling “netunium” is also accepted) in reference to the planet Neptune, which is the first planet in the solar system after Uranus, as the new element would come right after uranium. This way of naming a new element also served as a parameter for element 94, plutonium, since the (until then) planet Pluto orbits after Neptune.
By Stefano Araujo Novais
Chemistry teacher
Learn more about actin by clicking here. Learn its characteristics, properties, applications, obtaining and history.
Have you ever heard of the chemical element americium? Click here and learn about its characteristics, properties, obtainment and history.
Learn more about curium, its characteristics, properties, applications, production and history.
Learn about the particularities of the internal transition elements (actinides and lanthanides), which occupy the sixth and seventh period of group 3 of the Periodic Table.
Have you ever heard of the chemical element Laurentium? Click here and learn about its characteristics, properties, obtaining, precautions and history.
Learn a little more about mendelevium. Know its properties, characteristics, ways of obtaining and history.
Have you ever heard of the chemical element nobelium? Click here and find out about its characteristics, properties, methods of obtaining and history.
Have you ever heard of the chemical element protactinium? Click here and learn about its characteristics, properties, obtainment and history.
Learn more about thorium by clicking here. Learn its characteristics, properties, applications, obtaining and history.
Get to know uranium, as well as its properties, characteristics, ways of obtaining it, applications, risks and much more.

