THE oxidation and the reduction are reactions that occur when there is a electron transfer between chemical species. They are opposite reactions: in an oxidation there is a loss of electrons and in a reduction there is a gain of electrons.
These processes happen simultaneously, because when a substance donates electrons to another it is oxidized, while the substance that receives electrons undergoes reduction.
When a substance oxidizes, it causes the other species to reduce, hence the name of a reducing agent. Likewise, one substance reduces because of the oxidation of another and is therefore the oxidizing agent.
The oxidation and reduction reactions are demonstrated by the variation of the oxidation number (nox) of the species involved.
Generally, a reaction that presents oxidation and reduction can be represented by:
A + B+ → A+ + B
Where,
A: substance that undergoes oxidation, loses electrons, increases its value and is the reducing agent.
B: substance that undergoes reduction, gains electrons, decreases oxidation and is the oxidizing agent.
Examples of oxidation and reduction
See the following image for an example of the redox chemical equation.
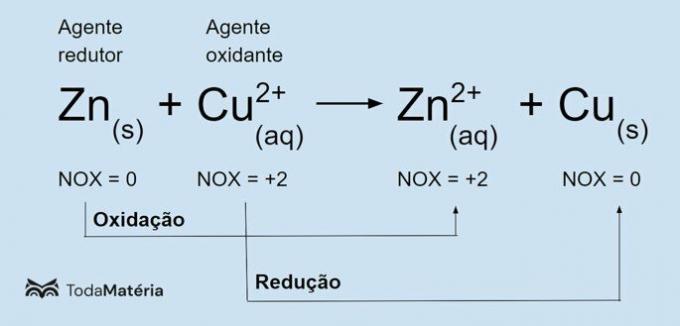
The metallic zinc undergoes oxidation and causes the reduction of copper, so it is the reducing agent. Its oxidation number (nox) increases from 0 to +2 because it loses 2 electrons.
The cupric ion (Cu2+) undergoes reduction and causes copper to oxidize, so it is the oxidizing agent. Its oxidation number (nox) decreases from +2 to 0 because it gains 2 electrons and becomes metallic copper, which is electrically neutral.
Other examples of redox reactions in everyday life are:
methane combustion
CH4(g) + 2O2(g) → CO2(g) + 2H2O(v)
Photosynthesis
6CO2(g) + 6H2O(v) → C6H12O6(here) + 6O2(g)
iron corrosion
2Fe(s) + 3/4O2(g) + 3 hours2O(v) → 2Fe (OH)3(s) (rust Fe2O3.3H2O)
Learn more about oxidation number (nox).
How do oxidation and reduction reactions occur?
The equation of the redox reaction involving copper and zinc can be represented by half-reactions, as shown below.
- oxidation half-reaction: Zn0(s) → 2e- + Zn2+(here)
- Reduction half-reaction: ass2+(here) + 2e- → ass0(s)
- global equation: Zn0(s) + ass2+(here) → Zn2+(here) + ass0(s)
This electron transfer in the redox reaction occurs according to the reduction potential of the species involved. The component with the lowest reduction potential has the tendency to donate electrons, while the one with the highest potential will receive them.
For example, zinc has a reduction potential of -0.76V, while copper has a reduction potential of +0.34V. Therefore, zinc, because it has a lower reduction potential, is a better reducing agent and promotes the reduction copper, which has a higher reduction potential and, therefore, gains electrons and causes zinc oxidation.
Also read about redox reactions.
Exercises on oxidation and reduction
Use the following questions to test your knowledge of what you have learned in this text.
question 1
Regarding oxidation and reduction reactions, it is correct to state that
a) The increase in the nox of the chemical species indicates that it has undergone a reduction.
b) The substance that loses electrons undergoes oxidation and is an oxidizing agent.
c) In redox reactions, electrons are transferred.
d) The substance that gains electrons is reduced and is a reducing agent.
e) The decrease in the nox of the chemical species indicates that it has undergone oxidation.
Correct alternative: c) In oxidation-reduction reactions, electron transfer occurs.
In redox reactions, electrons are transferred.
The substance that gains electrons undergoes reduction and is the oxidizing agent. The decrease in the nox of the chemical species indicates that it has been reduced.
The substance that loses electrons undergoes oxidation and is a reducing agent. The increase in the nox of the chemical species indicates that it has undergone oxidation.
question 2
Examples of redox reactions in everyday life EXCEPT
a) Corrosion
b) combustion
c) Photosynthesis
d) Neutralization
Incorrect alternative: d) Neutralization.
A neutralization reaction is one that occurs between an acid and a base, resulting in salt and water. For example:
NaOH + HCl → NaCl + H2O
Examples of a redox reaction are:
methane combustion
CH4(g) + 2O2(g) → CO2(g) + 2H2O(v)
Photosynthesis
6CO2(g) + 6H2O(v) → C6H12O6(here) + 6O2(g)
iron corrosion
2Fe(s) + 3/4O2(g) + 3 hours2O(v) → 2Fe (OH)3(s)
question 3
Observe the following oxidation-reduction reactions and indicate which are the oxidizing and reducing agents.
I. Zn0(s) + ass2+(here) → Zn2+(here) + ass0(s)
II. Ass2+(here) + H2(g) → 2H+(here) + ass(s)
III. Zn(s) + 2H+(here) → Zn2+(here) + H2(g)
Reply:
In a redox reaction, the one undergoing oxidation is the reducing agent and the one undergoing reduction is the oxidizing agent.
I. Zn0(s) + ass2+(here) → Zn2+(here) + ass0(s)
Oxidizing agent: copper (Cu)
Reducing agent: zinc (Zn)
II. Ass2+(here) + H2(g) → 2H+(here) + ass(s)
Oxidizing agent: copper (Cu)
Reducing agent: hydrogen (H)
III. Zn(s) + 2H+(here) → Zn2+(here) + H2(g)
Oxidizing agent: hydrogen (H)
Reducing agent: zinc (Zn)
Gain more knowledge with the contents:
- What are stacks?
- electrochemistry
- Electrolysis
Bibliographic references
FONSECA, M. R. M. Chemistry, 2. 1. ed. São Paulo: Attica, 2013.
SANTOS, W.L.P; MOL, G.S. Citizen chemistry, 3. 2. ed. São Paulo: Editora AJS, 2013.
USBERCO, J. Connect chemistry, 2: chemistry. - 2. ed. São Paulo: Saraiva, 2014.