Balancethermal is the condition in which a body finds itself in the sametemperature than their surroundings. It is observed that all bodies that are at higher temperatures than their neighbors tend to give them heat spontaneously until both start to present the same temperature.
Lookalso:Fundamentals of Thermology
Thermal equilibrium and the zero law of Thermodynamics
Thermal equilibrium is the central concept behind the zero law of thermodynamics. Such law establishes that, in the case where two thermodynamic systems, THE and B, are in thermal equilibrium with a third thermodynamic system, Ç, then, THE and B they will also be in thermal equilibrium.
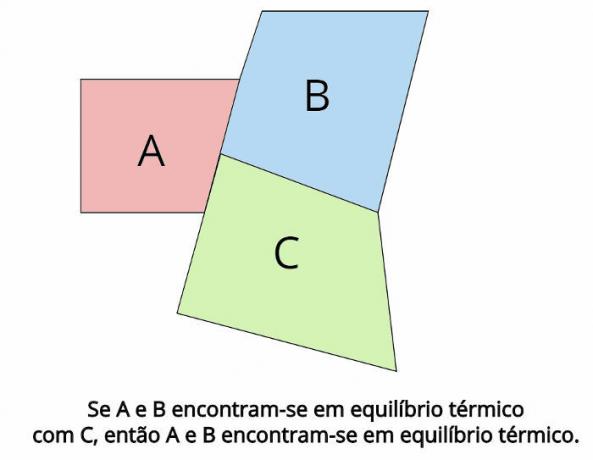
In thermal equilibrium, the final temperatures of each body must be equal: TTHE = TB = TÇ
Check out what the statement of the zero law of Thermodynamics establishes:
“If two bodies are in thermal equilibrium with a third body, then those bodies will be in thermal equilibrium with each other.”
Another way to understand thermal balance is based on the internal energy of bodies. Internal energy, or simply thermal energy, is a physical quantity
directlyproportional à temperature of the body. Therefore, if there are bodies with different temperatures within the same thermodynamic system, they will have different modules of internal energy and therefore will transfer some of that energy between them until there is no difference between their energies. internal. Do you want to know more about what internal energy is and what its properties are? Access the article: Internal energy.Do not stop now... There's more after the advertising ;)
heat and thermal balance
Heat transfer always occurs spontaneously, from the body with the highest temperature to the body with the lowest temperature. This transfer of energy in the form of heat can occur through processes such as driving, convection and radiation.
Driving: It is the transfer of heat between bodies that occurs especially in solids. In this type of conduction, no mass transfers occur. This type of heat transfer explains how thermal equilibrium occurs in metals, for example.
Convection: It is a heat transfer that takes place in fluids. In this mode of heat transfer, there is mass transfer, as the heated fluid moves, forming convection currents until all the fluid reaches thermal equilibrium.
Radiation: It is the transmission of heat through electromagnetic waves, therefore, this process occurs even if there is no physical medium between the body and another body at different temperatures. The heat that is transferred, in this case, is the equivalent of electromagnetic waves with less energy than visible light, thus being thermal radiation, located in the region of the infra-red.
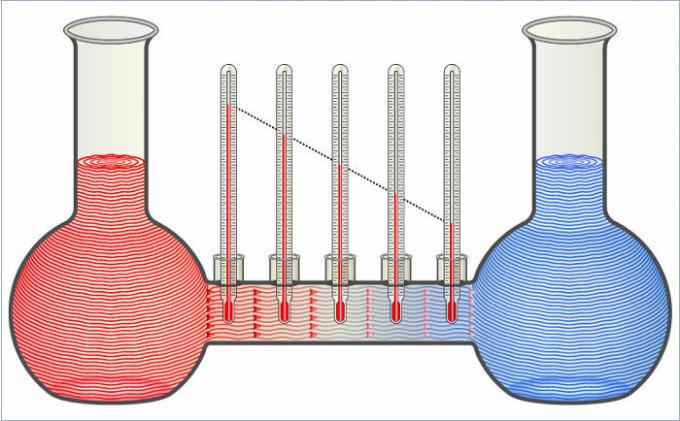
The two liquids in the figure transfer heat to each other until their temperatures are equal.
Do you want to know more about how each of the heat transfer processes takes place? Access the article: Heat propagation processes.
sensible heat
when there is differenceintemperature between two bodies, or between a body and its surroundings, there will be heat exchange between them spontaneously, so that the higher temperature body cools down, and lower temperature bodies heat up until they all reach the temperature in balancethermal.
The amount of heat that is exchanged between bodies at different temperatures is called sensible heat and this amount can be calculated from the formula shown in the figure below:
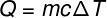
Q – heat (lime or J)
m – mass (g or kg)
ç - specific heat (cal/gºC or J/kg. K)
ΔT – temperature variation (°C or K)
In the formula shown above, it is important to highlight the greatness of the name specific heat. such magnitude measures the amount of energy per mass that a substance needs to yield, or absorb, to have its temperature varied by 1°C. In the case of pure water, for example, and under normal pressure conditions, to vary its temperature by 1ºC, 1.0 calorie is needed for each gram of water.
Thus, all substances that have established thermal contact with each other tend to reach the condition of balancethermal over time spontaneously, however, some require a greater amount of energy to do so and this directly affects the temperature to reach thermal equilibrium.
readalso:What is temperature?
latent heat
It is possible that during heat exchanges with its surroundings, a body presents pressure, temperature and volume that cause it to undergo a change in its physical state. These changes occur in temperatureconstant (for bodies composed of a single substance, without impurities), that is, despite receiving or giving heat to the external environment, the temperature of these bodies does not change.
This is only possible because all the energy exchanged, in this case, is being used to change the conformation of your molecules. From the moment the energy barrier is "overcome" and all the body's contents are in another physical state, the The body continues to exchange heat with its surroundings, unless, of course, its temperature is equal to the temperature outside.
O latent heat can be calculated from the formula shown in the figure below, check it out:
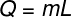
Q – latent heat (lime or J)
m – mass (g or kg)
L – specific latent heat (cal/g or J/kg)
Thermal Equilibrium Formula
In case we want to find out what is the temperatureinbalance of some thermodynamic system, it is necessary that we consider the system in question as a systemisolated, that is, we must assume that no amount of heat is exchanged with the neighborhoods of this system.
From this condition, we can say that the entire amount of heat exchanged is exchanged only between the bodies that make up this system, disregarding heat losses for the walls of the container, for example. In this case, we say that the container has thermal capacity negligible, ie it does not absorb any heat.
Imagine the following situation: in a cup of hot tea, with negligible heat capacity, pour some ice cubes. In order to determine the thermal equilibrium temperature, in addition to knowing the initial conditions of the system, we must make some considerations:
All the amount of heat that the hot tea gives to the ice will be fully absorbed by it, since the cup has negligible heat capacity.
We must disregard heat losses to the air and any other surroundings, so that this cup of tea can be understood as a closed thermodynamic system.
In this way, we can establish that the entire amount of heat received by the ice was given up by the hot tea, with that, we wrote our formula for calculating the thermal balance:
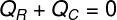
QR – Heat received
QÇ – heat given in
The heat given in (QÇ), refers to the amount of heat that the hot tea transferred to the ice cubes inserted in it. Already the heat received (QR) is the amount of heat these ice cubes received. This amount of heat will have two natures: heat sensitive and hot latent, since, to enter into thermal equilibrium, the ice cubes will likely melt.
Determining the thermal equilibrium temperature
Let's determine the thermal equilibrium temperature from the following situation:
A cup, with negligible thermal capacity, which contains 200 ml (200g) of tea at an initial temperature of 70°C, receives 10g of ice at a temperature of -10°C. Determine the thermal equilibrium temperature of the system (assume that the specific heat of tea is equal to the specific heat of water):
Data:
çWATER = 1.0 cal/g°C
çICE = 0.5 cal/g°C
LICE = 80 cal/g
First, we consider that all the heat received by the ice was given away by the tea:

Next, it is necessary to detail which forms of heat were given and received:
Tea: The tea gave only sensible heat (Qs), as his physical condition has not changed.
Ice: The ice was initially at -10°C, so it received sensible heat (Qs) until a temperature of 0 ºC, then received latent heat (QL) to liquefy. After becoming liquid, it received latent heat (Qs) until it enters thermal equilibrium (TF) with tea.
Translating what was analyzed above in the form of an equation, we will have the following calculation to solve:
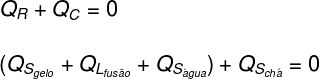
Replacing the data provided by the exercise in the equation found above, we will have to solve the following calculation:
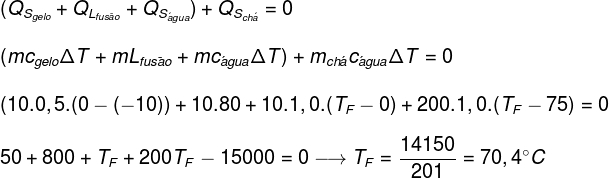
According to the calculation made above, the equilibrium temperature of the tea+ice system should be approximately 70.4°C.
Thermal balance experiment
To test the thermal balance between two bodies, we can perform several experiments. The simplest of these, however, involves the use of a calorimeter it is a thermometer. The calorimeter is an adiabatic container (which does not allow the passage of heat), with thermal capacity aboutnegligible, like a pot lined with styrofoam, for example, which is a good thermal insulator.

The calorimeter is used to measure the temperature variation of the system inside.
Thermal balance and life on Earth
O balancethermal it plays a fundamental role in terrestrial life. Without the presence of greenhouse gases in the Earth's atmosphere, most of the thermal radiation of the planet would leave it, propagating into space. Over time, this would cause massive cooling across the planet, causing the oceans to freeze over time.
In addition, the oceans play a key role in balancethermal of the planet. By virtue of its great pasta and heatspecific, the oceans are endowed with a huge capacitythermal, that is, they need to receive enormous amounts of heat to change their temperature. For this reason, they are able to regulate the planet's temperature very efficiently. Regions far from the oceans and with little water usually present large thermal ranges, as in the case of deserts, which are extremely hot during the day and freezing at night.
Therefore, the balancethermal it is a process of fundamental importance for the maintenance of the planet's physical, chemical and biological processes and, therefore, essential for the existence of life on Earth.
By Me. Rafael Helerbrock