The text First law of radioactivity or Soddy's first law showed the first general law that corresponds to what happens when an atom of a radioactive element undergoes alpha decay.
THE second law of radioactivity or Soddy's second law refers to beta decay. See what this law says:
“When an atom emits a beta particle, its atomic number (Z) increases by one unit and its mass number (A) remains the same.”
Generically, we can represent this law through the following equation:
ZTHEX →-10β + Z+1THEY
The atomic number (Z) is the number of protons in the atomic nucleus. The mass number (A) corresponds to the sum of the protons and neutrons existing in the nucleus (A = p + n). This means that the obtained atom is an isobare of the original atom, that is, they have the same mass number.
Here's an example: thorium-231 emits a beta particle and forms protactin-231:
23190Th→ -10β + 23191Pan
Note that there is conservation of the mass number and the atomic number in the two members of the equation:
A: 231 = 0 + 231;
Z: 90 = -1 + 91.
So you can use this rule to find out which particle was emitted or which atom was formed.
As explained in the text Beta issue (β), this emission is like an electron because it has a charge of -1 and has no mass. But then why does the atomic number increase and the mass number remain constant?
This fact was explained by a hypothesis launched by the Italian physicist Enrico Fermi (1901-1954).

A postage stamp printed circa 2001 in the USA shows an image of Nobel Prize winner in Physics Enrico Fermi
Enrico Fermi proposed the following:
“Beta particle emission occurs when an unstable neutron inside the atomic nucleus disintegrates, forming a proton that remains in the nucleus. At the same time this disintegration forms the beta particle (-10β), which is similar to the electron and is emitted by the nucleus together with the gamma radiation (γ - it's just electromagnetic radiation, having no electrical charge and no mass) and a neutrino (00ν, charge particle and null mass).”
I.e:
01n → 11p + -10β + 00 γ + 00ν
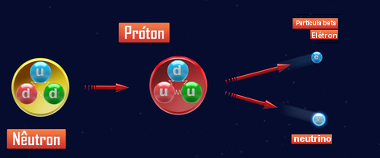
Neutron disintegration for beta particle emission
The proton and the neutron have practically the same mass, which is why, when an atom emits a beta particle, its number of mass (A) remains the same, ie while the neutron disintegrates, the proton is formed, replacing it in the nucleus, so to say. Since the proton is formed, the atomic number increases by one.
See the following illustration for another example of how this law actually applies in cases of beta decays. In it, the isotope 14 of the element carbon emits a beta particle, transforming itself into nitrogen-14:

Beta decay of carbon-14 that generates nitrogen-14
__________________
* Image copyrighted: catwalker / Shutterstock.com.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/segunda-lei-radioatividade-ou-segunda-lei-soddy.htm

