THE rutherfordium is a synthetic element with atomic number 104, belonging to group 4 of the Periodic table, being the first member of the transactinide series. Its first detection dates from 1964, in the famous laboratories of the city of Dubna. Like other transactinides, the official name of element 104 was involved in a conflict between the Soviets and the Americans, in a Cold War piece in the history of chemistry.
the rutherfordium has no practical applications, given that its most stable isotope has about two and a half hours of half life. However, studies in gaseous systems and solutions prove its chemical similarity with the other elements of group 4, such as zirconium and hafnium.
Read too: Seaborgium — synthetic element named after scientist Glenn Seaborg
Summary of Rutherfordium
- It is a synthetic chemical element located in group 4 of the Periodic Table.
- It was first synthesized in 1964 at the Joint Institute for Nuclear Research in Dubna, Russia.
- It is a radioactive elementgrandmother.
- Like other transactinides, rutherfordium suffers from low stability and it is difficult to synthesize considerable samples for studies.
- Its name was made official only in 1997, after several years of dispute between the Americans and the Soviets.
Rutherfordium properties
- Symbol: Rf
- atomic number: 104
- atomic mass: 267 c.u.s.
- Electronic configuration: [Rn] 7s2 5f14 6d2
- most stable isotope: 267Rf (2.5 ± 1.5 hours half-life)
- chemical series: group 4, transactinides, superheavy elements
Features of Rutherfordium
Like all transactinides, i.e. elements just after laurence (Lr), rutherfordium is a radioactive element. Its most stable isotope was detected in 2004 and its half-life (the time required for the amount of the radioisotope fall by half) is two and a half hours, with a margin of error of one and a half hours, more or less.
The great difficulty in establishing the chemical characteristics of rutherfordium and other transactinides is, in general, the fact that there is a low rate of production, either in quantity or in velocity. In these elements, for example, it is very common to chemically evaluate only a single atom, which, in a way, requires adaptations in terms of calculations, since most of the equations are established for systems with more than one atom. Furthermore, often the isotopes have very short half-lives, which makes more in-depth studies difficult or even impossible.
In the specific case of Rf, scientists have already managed to prove that its behavior in liquid phase is similar to that of other elements. lighter group 4, zirconium and hafnium, as in the formation of fluorides in solution with subsequent extraction in ion exchange resins. This behavior helped solidify the presence of rutherfordium in group 4 of the Periodic Table.
Read too: New chemical elements — the four missing elements in the 7th period
Obtaining Rutherfordium
Transactinides need a large infrastructure for their production. All are synthesized with particle accelerators, in which ionic species collide with heavy elements. The detection of these elements is also not simple and straightforward.
When formed, the radioactive element, by its nature, begins to decay and exhibit emissions, such as alpha and beta particles. It is often necessary to assess the radioactive decay of the atom formed or even identify atomic species that may arise from these nuclear reactions, as in a puzzle.
Add this to the fact that the half-lives of transactinide isotopes are often short, in the range of seconds, making it only possible to obtain an amount in the range of a few atoms or even a single atom.
In the case of Rf, the first synthesis reported for this element involved the collision of plutonium isotopes, Pu, with ions of neon isotope 22, Huh.
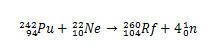
However, other isotopes of rutherfordium can be produced by modifying the species that will collide. For example, isotope 261 can be produced by the reaction between oxygen-18 and curium-248, producing five neutrons.
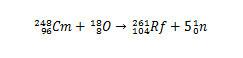
Check it out on our podcast: How does a particle accelerator work?
Precautions with Rutherfordium
Since rutherfordium cannot even be produced on a considerable scale, the risks associated with this element are linked to the effects of radiation. However, in a controlled laboratory, these risks are anticipated and thus minimized.
History of Rutherfordium

All transactinides were involved in a race for their discovery in the 1960s and 1970s. This episode is reported as the War of Transfers, a piece of the Cold War in the history of chemistry. Despite being called that, which suggests that the disputes occurred over elements after fermium, an element atomic number 100, the elements directly involved were 104 to 109, the newly discovered transactinides.
In the case of rutherfordium, the dispute began in 1964., when Soviet researchers at the Joint Institute for Nuclear Research in the city of Dubna Russia, reported the discovery of element 104 isotope 260 by bombarding plutonium-242 with ions neon-22. But the Dubna researchers only presented one piece of evidence, which was the detection of an isotope which has spontaneously decayed, without clearly identifying its mass and its time of half life. As a result, the discovery was viewed with suspicion.
Five years later, in 1969, a team of American scientists at Lawrence Berkeley National Laboratory in Berkeley, California, led by Albert Ghiorso, said he provided sufficient evidence for the discovery of element 104 isotope 257 by colliding californium-249 with carbon-12. The same scientists were later able to produce the isotope 259 of element 104. In 1973, scientists at Oak Ridge National Laboratory independently confirmed the atomic number 104 for the mass 257 isotope produced at Berkeley.
The following years were of great dispute between the scientists of the two countries, until, in 1985, the of Pure and Applied Chemistry (IUPAC) and the International Union of Pure and Applied Physics (IUAP) form a mixed commission of nine scientists, called the Transfers Working Group (Transfermium Working Group or TWG). The TWG was created to decide who was actually responsible for the discoveries of elements ranging from 101 to 112.
Even so, the TWG's decisions were not always without question. In the case of element 104, the working group decided that the Credit should be shared between Soviet and American scientists, something that Berkeley scientists did not like at all.
In 1991, Ghiorso and Seaborg, from the Berkeley team, even claimed that the identification of element 104 by the Dubna scientists was clearly wrong and, at one point, denied the validity of TWG's conclusions, considering them a disservice to the community scientific.
No wonder, in In the early 1990s, the names for the new elements were still not a consensus.. There were then negotiations involving German, Russian and American scientists, which proved to be frustrated. There, in 1992, the German laboratory Gesellschaft für Schwerionenforschung (GSI) proposed names for elements 102 through 109, putting meitnerium for element 104.
Even so, the list, despite being praised, was not accepted by the scientists involved. The decision could be taken in 1994, during the Iupac Inorganic Compound Nomenclature Commission (CNIC) conference. In it, the name dubnium was chosen for element 104, however, the American Chemical Society adopted, in the same year, the name rutherfordium for the element 104. element 104, in a moment of discredit on the part of the Americans, who came to question the authority of the IUPAC to formalize new names.
only in 1997, at the IUPAC general conference in Geneva, is that element 104 was finally made official as rutherfordium, after the ACS gave way in the nomenclature of other nearby elements.
Exercises solved on rutherfordium
question 1
Rutherfordium is a synthetic element and one of the main difficulties in studying it is the fact that it is not possible to synthesize large amounts of it.
Among the possible factors that contribute to this difficulty, we can indicate:
(A) Rutherfordium has long half-life isotopes of the order of millions of years.
(B) Rutherfordium decays spontaneously and very rapidly, preventing detection of its macroscopic quantities.
(C) There are no technologies capable of synthesizing rutherfordium, their data being strictly theoretical and without scientific basis.
(D) The laws of chemistry state that it is impossible to synthesize elements whose atomic number exceeds that of lawrence, 103.
(E) During the synthesis of rutherfordium, lighter elements of its group are chemically prioritized.
Reply: letter B
Transactinide elements, such as Rf, are radioactive and spontaneously decay at high speed, since their half-life is short. Thus, at the end of the experiment, few atoms are left of the synthetic species.
question 2
In 1964, researchers from Dubna claimed to have synthesized the rutherfordium isotope-260 (Z = 104). In the case of this isotope, what is the number of neutrons?
(A) 104
(B) 260
(C) 151
(D) 156
(E) 161
Reply: letter D
The number of neutrons (n) can be calculated, using the mass number (A) and the atomic number (Z), through the following equation:
A = Z + n
Substituting, we have:
260 = 104 + n
n = 260 - 104
n = 156
image credits
[1] roseed abbas / shutterstock
By Stefano Araújo Novais
Chemistry teacher
Source: Brazil School - https://brasilescola.uol.com.br/quimica/rutherfordio-rf.htm
