THE battery is a system where the oxidation-reduction reaction takes place. In this device, the chemical energy produced in the spontaneous reaction is converted into electrical energy.
Oxidation and reduction reactions occur simultaneously in a cell. When one species undergoes oxidation, it donates electrons to the other species, which, upon receiving them, undergoes reduction.
Therefore, the one undergoing oxidation is the reducing agent and the one undergoing reduction is the oxidizing agent.
THE oxidation occurs when a species loses electrons and becomes a cation: A → A+ + and-.
THE reduction occurs when a species gains electrons and becomes electrically neutral: B+ + and- → B.
In the chemical equations, this electron transfer is demonstrated by the change in the oxidation number (nox).
Reduction reactions occur inside the cells and the electric current arises with the migration of electrons from the negative to the positive pole.

How does a stack work?
One redox reaction can generally be represented by the equation:
A + B+ → A+ + B
Where,
A: substance that undergoes oxidation, loses electrons, increases its value and is the reducing agent.
B: substance that undergoes reduction, gains electrons, decreases oxidation and is the oxidizing agent.
See in the following image how this process can be represented.
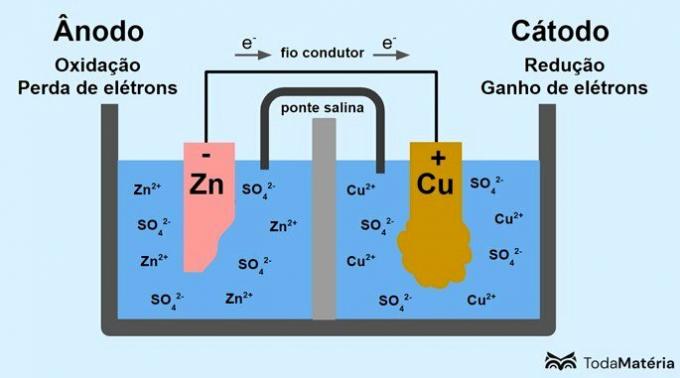
The system divided into two semicells and formed by two metallic electrodes connected externally by a conducting wire was developed by John Frederic Daniell (1790-1845) in 1836.
The battery consists of two electrodes, connected by a conducting wire, and an electrolyte, where the ions are. The electrode is the conducting solid surface that allows the exchange of electrons.
anode: electrode at which oxidation occurs. It is also the negative pole of the battery.
Cathode: electrode at which reduction occurs. It is also the positive pole of the battery.
In the image above, metallic zinc is the anode and undergoes the oxidation. Metallic copper is the cathode and undergoes reduction. The migration of electrons (e-) occurs from the anode to the cathode through the conducting wire.
The reactions that occur in the image system are:
- anode (oxidation): Zn(s) → Zn2(here) + 2e-
- Cathode (reduction): Cu2+(here) + 2e- → ass(s)
- general equation: Zn(s) + ass2+(here) → ass(s) + Zn2+(here)
Zinc is a metal with a greater tendency to lose electrons and, therefore, cations are formed in the solution. The zinc electrode begins to wear out and lose mass because zinc is released into solution when forming Zn cations2+.
The electrons from the anode arrive at the cathode and the metal cations, upon receiving them, are transformed into metallic copper, which is deposited on the electrode and increases its mass.
The salt bridge is an ionic current responsible for the circulation of ions in the system to keep it electrically neutral.
Also read about oxidation number (nox).
battery types
In a cell, the tendency of chemical species to receive or donate electrons is determined by the reduction potential.
The component with the highest reduction potential tends to undergo reduction, that is, to gain electrons. The species with the lowest reduction potential and, consequently, the highest oxidation potential, tends to transfer electrons.
For example, in the redox reaction Zn0(s) + ass2+(here) → ass0(s) + Zn2+(here)
Zinc oxidizes and donates electrons because it has a reduction potential E0 = -0.76V, less than the reduction potential of copper E0 = +0.34V and, therefore, it receives electrons and undergoes reduction.
See below for other examples of stacks.
Zinc and hydrogen stack
Oxidation half-reaction: Zn(s) → Zn2+ + 2e- (AND0 = -0.76V)
Reduction half-reaction: 2H+(here) + 2e- → H2(g) (AND0 =0.00V)
Global equation: Zn(s) + 2H+(here) → Zn2+(here) + H2(g)
Stack representation:
Copper and hydrogen cell
Oxidation half-reaction: H2(g) → 2H+(here) + 2e- (AND0 = 0.00V)
Reduction half-reaction: Cu2+(here) + 2e- → ass(s) (AND0 = +0.34V)
Global Equation: Cu2+(here) + H2(g) → 2H+(here) + ass(s)
Stack representation:
Get more knowledge on the topic with the contents:
- electrochemistry
- Electrolysis
Bibliographic references
FONSECA, M. R. M. Chemistry, 2. 1. ed. São Paulo: Attica, 2013.
SANTOS, W.L.P; MOL, G.S. Citizen chemistry, 3. 2. ed. São Paulo: Editora AJS, 2013.
USBERCO, J. Connect chemistry, 2: chemistry. - 2. ed. São Paulo: Saraiva, 2014.