To understand why gasoline is capable of generating vehicle movement, it is necessary to know more about its composition.
Hydrocarbons: they are the combustible liquid and are responsible for generating energy, all hydrocarbons are capable of combustion. Below is an image to make your understanding easier:
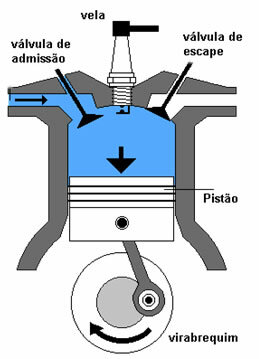
The reaction starts inside the combustion engine (represented by the figure above), with the presence of a mixture of fuel and air. This mixture is injected through the inlet valve and is stored on a piston that moves up and down.
When the piston moves upward, it compresses the mixture of air and gasoline. The spark plug fires and ignites the mixture.
The burning mixture generates hot gases that expand and produce a force that causes the piston to lower again, driving the crankshaft.
The exhaust valve opens and the gases are expelled by the rising piston.
The described process corresponds to the “four stroke” engine operation.
Do not stop now... There's more after the advertising ;)
By Líria Alves
Graduated in Chemistry
Brazil School Team
See more: Why does gasoline pollute?
What is the fuel that most pollutes the atmosphere?
fuels - Chemistry - Brazil School
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Gasoline: Hydrocarbon Explosion"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/gasolina-explosao-hidrocarbonetos.htm. Accessed on July 27, 2021.