the texts Oil Exploration and Extraction and How is oil transported? showed how oil is found, removed from underground deposits and transported. This transport through pipelines or supertankers (large ships) is carried out to carry the oil to the refineries. These are places where physicochemical processes are carried out for the oil refining gross (or oil refinement) to get the calls petroleum fractions.
Oil is not used directly in crude form, so it needs to go through this process. This fuel is composed of a complex mixture of hydrocarbons, plus small amounts of other classes of organic compounds that contain nitrogen, oxygen and sulfur. It is not possible to isolate each of these organic compounds that make up oil, as many have very close boiling points. Therefore, petroleum refining isolates fractions, that is, groups with a smaller number of organic compounds, mainly hydrocarbons that have similar molar masses.
Therefore, the basic difference between these oil fractions is the amount of carbon atoms present in their molecules. For example, gasoline is a fraction of petroleum formed by hydrocarbons that have 6 to 10 carbon atoms, while another fraction of petroleum, oil
diesel, has molecules with 15 to 18 carbons. See the image below for other petroleum fractions and the amount of carbon atoms in each case, in addition to the wide applications of these fractions in our society: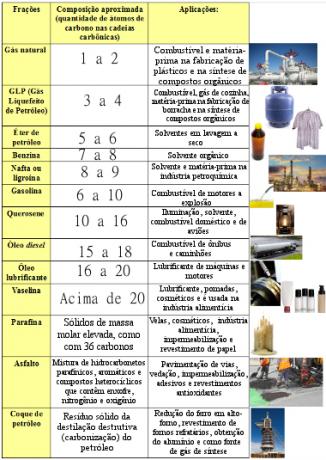
Main oil fractions, their approximate compositions and applications
The greater the molar mass of an organic compound, the higher its boiling point, that is, the temperature that changes from the liquid state to the vapor state. This property is important, because one of the main techniques used in refineries is the fractional distillation of oil, and it relies on this property to separate the oil fractions.
Mind Map: Oil

*To download the mind map in PDF, Click here!
Basically, this technique consists of placing the oil in an oven or furnace to be heated to around 400ºC. This furnace is coupled to a distillation tower the atmospheric pressure (or fractionation tower) that has several distillation dishes or trays (maximum 50 trays). As it goes up, each tray has a different temperature that decreases accordingly.
Do not stop now... There's more after the advertising ;)
Thus, the following occurs, hydrocarbons with higher molar masses (of molecules with greater amounts of carbon) form a fraction that remains at the bottom of the container in the liquid state, as its boiling point is very high. The fractions with lower molar masses and which have lower boiling points, on the other hand, go to the vapor state and go up in the distillation tower, reaching the next tray. If the temperature in this tray is lower than the fraction's boiling point, it condenses and is retained. If not, it continues going up to the next tray and so on, until the lightest fraction reaches the last dish in the column, where the temperature is the lowest and is collected there.
This process is repeated again to ensure the efficiency of the process. In this part of the process, gas, gasoline, naphtha and kerosene are mainly obtained.
The high molar mass liquid that remained at the bottom of the distillation tower is then forwarded to another furnace to be heated again. But, it turns out that this furnace is coupled to a vacuum distillation tower or reduced pressure, that is, it has a vapor pressure lower than atmospheric pressure. Thus, these heavy compounds boil at temperatures lower than 400ºC, which prevents their molecules from decomposing, that is, being broken. The same process that took place in the other distillation tower occurs again and new fractions of this residue are obtained. These petroleum fractions are heavier than those obtained in the first distillation. In this part of the process, mainly grease, paraffins, lubricating oils and bitumen are obtained, which is used for making asphalt and waterproofing.
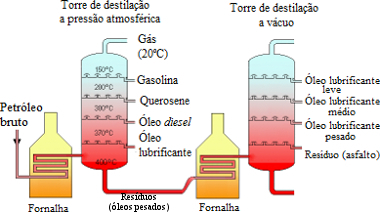
Scheme of some oil fractions obtained by fractional distillation, the first stage of its refining*
For greater use of oil, after refining it can go through a process called cracking. See about this next step in the following text:
→ oil cracking
__________________________
* Image credits (modified): Author: Theresa knot/ Source: Wikimedia Commons
* Mind Map by Me. Diogo Lopes
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Oil Refinement"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/refinamento-petroleo.htm. Accessed on June 27, 2021.