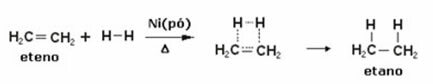
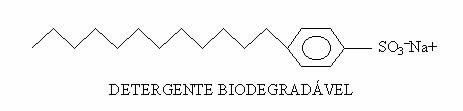
For anyone who has already suffered from one of the worst pains in the world, the famous kidney colic, it would be interesting to know how kidney stones form. Know that this problem that affects a large part of the population has its explanation in the chemical composition of stones found in affected kidneys.
Our urinary system continuously and daily excretes the chemical substances calcium phosphate and calcium oxalate. The problem arises when our urine becomes saturated and does not properly excrete these substances. The stones appear in response to the accumulation of phosphate and calcium oxalate in the kidneys.
The appearance of kidney stones is a natural process of precipitation of insoluble salts. The process is slow and takes about two to three years for a kidney stone to form.
Do not stop now... There's more after the advertising ;)
To avoid crystallization of oxalate and calcium phosphate salts, it is recommended to ingest 2 liters of water daily.
By Líria Alves
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
SOUZA, Líria Alves de. "Precipitation of insoluble salts"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/precipitacao-sais-insoluveis.htm. Accessed on July 27, 2021.