At combustion reactions they are present in many aspects of our daily lives. For example, the main sources of energy generation result from the combustion or burning of certain fuels, such as ethanol, gasoline, charcoal, among others. Furthermore, the energy we need to survive and to do work is the result of combustion reactions that take place within our cells when we “burn” the food we eat.
But what does it take to have a combustion reaction?
Three things are needed:
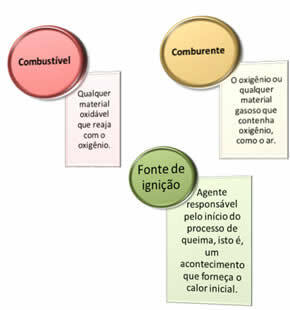
These three factors make up the fire triangle, because once the combustion reaction begins, the energy released sustains the reaction and allows it to continue until the fuel, oxidizer, or heat (released energy) is gone. This means that there will be a Chain reaction.
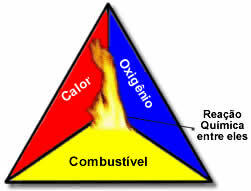
For example, if someone throws a lit cigarette in a forest, there will be a fire (combustion reaction). In this case, the Bush it's the fuel, O oxygen present in the air atmospheric is the oxidizer it's the cigarette lit was the ignition source. This burning will continue until one of the three factors is eliminated. If firefighters throw water, the heat will be eliminated. But even if nothing is done to stop this burning, it will end at some point, that is, when the fuel (forest) runs out.
As already mentioned, in these reactions heat is released, thus combustion is an exothermic reaction. However, this type of reaction is incomplete combustion. To understand why, see the difference between complete and incomplete combustion:
Complete combustion:
Analyzing organic compounds as fuels, we have to:
| THE complete combustion it will occur when the carbon chain is broken and all carbon atoms in the carbon chain are completely oxidized. |
| You formed products by hydrocarbons will be the CO2 (carbon dioxide) and H2O (Water). |
Observe the complete combustion of isooctane, which is one of the components of gasoline.
Ç8H18(g) +25/2 O2 (g) → 8 CO2(g) + 9 hours2O(1)
incomplete combustion
| In this case, there is not enough oxidizer, that is, enough oxygen to burn all the fuel. |
| Thus, the products formed are CO (carbon monoxide) and H2O. |
Observe the same combustion of isoctane, however, now incompletely:
Ç8H18(g) + 17/2 O2(g) → 8 CO (g) + 9 hours2O(1)
The burning of forests is an example, as the resulting emissions consist of CO and particular matter, such as soot (C), as well as ash and other simple and complex organic compounds. Nitrogen oxide, ozone and aldehydes can also be formed as a result of secondary reactions due to the presence of other components in the air.
Ç8H18(g) + 9/2 O2 (g) → 8C (g) + 9 hours2O(1)
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
Source: Brazil School - https://brasilescola.uol.com.br/quimica/combustao-completa-incompleta.htm

