One polar compound (or substance) is one that has two regions with different electron densities. One of these regions has a positive character (white area), and another has a negative character (yellow area), as we can see in the following representation:

Representation of regions of different charge in a polar compound
Know if a certain composite is polar implies knowing the type of intermolecular force that favors the interaction between its molecules or with molecules of other substances, as well as making assumptions about their solubility and melting points and boiling.
For example: with regard to solubility, polar compounds have a good ability to dissolve into polar compounds. As for intermolecular forces, depending on the case, polar compounds can interact by forces permanent dipole or hydrogen bonds (strength which also results in higher melting points and boiling).
Here are two practical ways to determine whether a compound is polar or not.
Determination of polarity through the number of clouds and number of ligands
We can determine if a composite is polar by the relationship between the number of equal atoms attached to the central atom and the number of electron clouds in that central atom.
Note: An electron cloud is any chemical bond between two atoms, or a pair of electrons from the valence shell of an atom that are not participating in a bond.
If the number of clouds present in the central atom is different from the number of equal ligands in that central atom, we have a polar compound. To better understand, follow the examples below:
1st Example: Hydrocyanic Acid Molecule
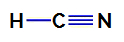
Structural formula of hydrocyanic acid
In hydrocyanic acid, the central atom is carbon, which has four electrons in its valence layer for belonging to the IVA family of the periodic table. How carbon is making a single bond (sharing two electrons, with one electron from each atom involved) with hydrogen and a triple bond with nitrogen, so there are no non-bonding electrons in the atom central.
Thus, in hydrocyanic acid, there is the presence of two electronic clouds (a single bond and a triple bond) and a ligand equal to the other. Therefore, it is a polar compound.
2nd Example: Ammonia molecule (NH3)
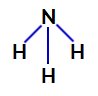
Ammonia structural formula
In ammonia, the central atom is nitrogen, which has five electrons in its valence shell as it belongs to the VA family of the periodic table. As nitrogen is making a single bond (sharing of two electrons, with one electron of each atom involved) with each hydrogen atom, two of its five electrons do not participate in bonds.
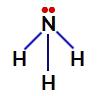
Non-nitrogen-binding electrons in ammonia
Thus, in ammonia, there are four electron clouds (three single bonds and the non-bonding electron pair) and three equal ligands (the three hydrogens). So it's a polar compound.
Determination of polarity through the dipole moment vector of a compound
We can determine if a composite is polar by the analysis of the resulting dipole moment vector in its structural formula, taking into account its molecular geometry and the difference of electronegativity between the atoms involved.
Note: Descending order of electronegativity of the elements: F > O > N > Cl > Br > I > S > C > P > H.
When the sum of the vectors present in the molecule is different from zero, the compound will be polar. To better understand, follow the following examples:
1st Example: trichloromethane molecule
Trichloromethane is a compound that presents tetrahedral geometry, as we can see in its structural formula below:

Structural formula of trichloromethane
To find out whether or not it is a polar compound, we must initially place the dipole moment vectors (arrows that indicate which atom is more stable than the other) in the structural one, as in the following example:
Note: Chlorine is a more electronegative element than carbon. In turn, carbon is a more electronegative element than hydrogen.
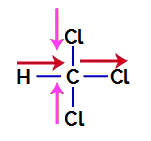
Dipole Moment Vectors in Trichloromethane
Vectors in pink can be represented by +x and -x, as they have the same direction (vertical) and opposite directions (up and down). Vectors in red are represented by +x, as they have the same direction and the same direction. Thus, the resulting dipole moment vector (sum of the vectors) is represented by:
μr = (+x) + (-x) + (+x) + (+x)
μr = +X – x + x + x
μr = 2x
Since the resulting dipole moment vector is nonzero, we have a polar compound.
2nd Example: water molecule
Water is a compound that presents angular geometry, as we can see in its structural formula below:
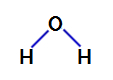
Structural formula of water
To find out whether or not it is a polar compound, we must initially place the dipole moment vectors (arrows that indicate which atom is more stable than the other) in the structure, as shown below:
Note: Oxygen is a more electronegative element than hydrogen.
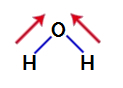
dipole moment vectors in water
Since the two vectors in the structure of water are diagonally across, we must use the parallelogram rule. In this rule, when we link the bases of the vectors, we have the creation of a resulting vector (which replaces the two used previously), as in the following model:
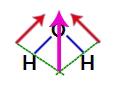
Resulting vector in the structural formula of water
As the water molecule has a single vector, therefore, the resulting dipole moment vector is non-zero, that is, we have a polar compound.
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-um-composto-polar.htm


