The compounds that involve this group are not found free in nature, therefore, obtaining methods that were developed in the laboratory are necessary. The three main methods of preparation of alcohols will be identified and explained below:
1st) Reduction of aldehydes, ketones and carboxylic acids: Reduction is a reaction against oxidation, in which hydrogen gas (H2) is used or a hydrogen derived from Zn and HCl or from Zn and acetic acid can be used.
Examples:
*Aldehyde:
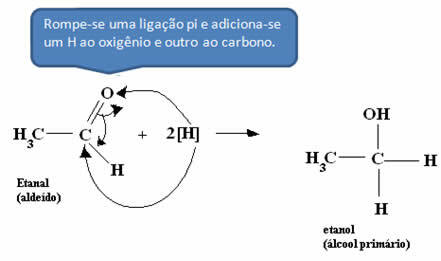
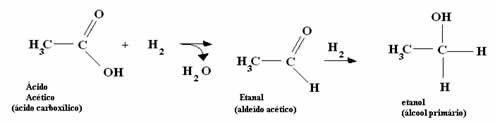
*Acetic Acid: The acid is first converted into aldehyde and, subsequently, the process mentioned in the previous item is carried out.
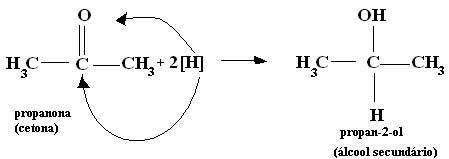
*Ketone: Since this functional group is, by definition, between two carbons, its reduction will form secondary alcohols.
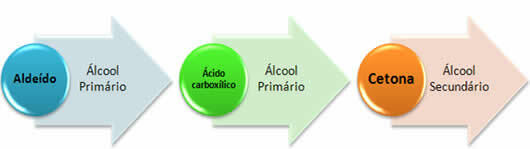
In short, we have:
2) Hydration of alkenes: Another way to produce alcohols is a reaction between alkene and water in an acidic medium, as shown in the example below:
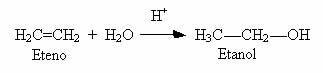
Only when you start with ethylene do you get a primary alcohol, all the others are secondary.
3rd) Grignard's Synthesis: Grignard compounds are compounds of the type:
RMgX or ArMgX where: R=alkyl
ar= arila
X= halogen
These compounds are very reactive, due to ionization:
RMgX → R- + MgX+
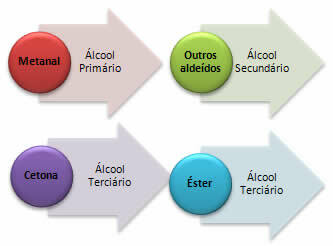
The R- ion is called carbanion and he makes a nucleophilic attack to another organic molecule that has a carbonation, that is, a positive carbon. Thus, in contact with aldehydes, ketones and esters, primary, secondary and tertiary alcohols are formed:
Examples:
*Aldehydes:
a) Metal – produces a primary alcohol:

b) All other aldehydes, other than methanol, will produce secondary alcohol:
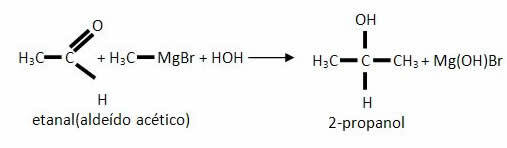
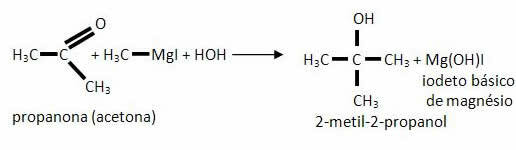
*Ketone: Tertiary alcohol will be obtained.
*Ester: The carbonyl of the esters reacts with the Grignard compounds to form a ketone, but it does not remain in this compound because its reactivity is greater than that of the initial esters, so the reaction continues as shown in the last item, for the formation of an alcohol tertiary.
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
| Alcohols are organic compounds that have a hydroxyl or oxidryl (OH) group attached to a saturated carbon atom. |
Source: Brazil School - https://brasilescola.uol.com.br/quimica/metodos-preparacao-dos-alcoois.htm
