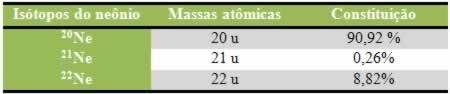
Osmosis is the movement of water that takes place inside cells through a semi-permeable membrane.
In this process, the water molecules move from a less concentrated medium to a more concentrated medium.
Therefore, osmosis serves to balance the two sides of the membrane, causing the solute-rich medium to be diluted by the solvent, which is water.
How does osmosis occur?
Osmosis is considered a passive transport, as in the passage through the membrane no energy is wasted.
In the process of osmosis, water, which is the solvent, tends to cross the semi-permeable membrane in order to balance the concentration of the solution. This action is carried out until the osmotic pressure is stabilized.
Therefore, the water moves from the less concentrated region to the more concentrated, naturally.
The passage of water from one medium to another is made in cells with the help of transporting proteins in the membrane, aquaporins. Thus, osmosis occurs whenever there is a difference in concentration between the cell's external and internal environment.
The result of osmosis is used in the nutrient exchange processes of animal and plant cells.
Also read about Passive Transport and Active Transport.
Hypotonic, isotonic and hypertonic solution
As we have seen, the osmosis process aims to equalize the concentrations of solutions, until an equilibrium is reached. For this we have the following types of solution:
- hypertonic solution: presents higher osmotic pressure and solute concentration.
- hypotonic solution: presents lower osmotic pressure and solute concentration.
- isotonic solution: the solute concentration and the osmotic pressure are equal, thus reaching equilibrium.
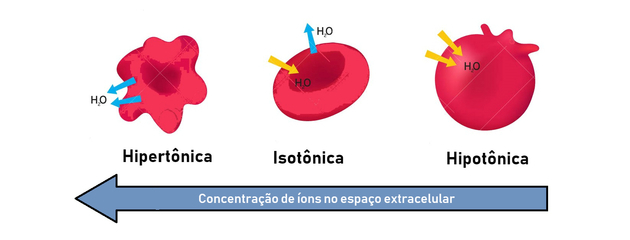
Therefore, osmosis occurs between a hypertonic (more concentrated) and hypotonic (less concentrated) way to generate a balance.
examples of osmosis
In cells, the plasma membrane is an envelope formed by a lipid bilayer, which hinders the movement of water in the cell. However, there are proteins specialized in its structure, aquaporins, which act as channels that facilitate the passage of water molecules.
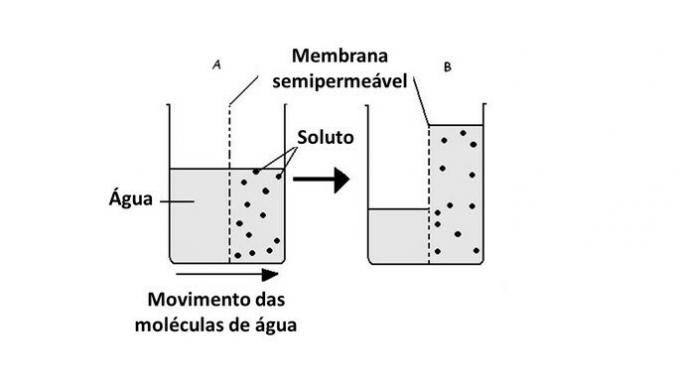
In a hypertonic environment, cells tend to shrink as they lose water. On the other hand, a cell placed in a hypotonic medium can swell until it breaks, as there is movement of water into the cell.
See below how osmosis occurs in animal and plant cells.
Osmosis in animal cell
when a animal cell, like red blood cells, are exposed to media with different concentrations, the movement of water in the cell occurs as follows:
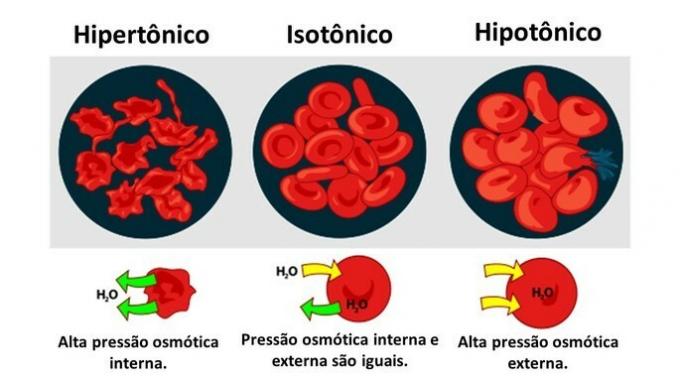
When the medium is rich in solute, a solution that is hypertonic with respect to the cytoplasm, cells lose water to the medium and wither.
When the medium is poor in solute, a hypotonic solution, water molecules tend to enter the cell and, although the membrane is resistant, depending on the amount, disruption can occur.
Plant cell osmosis
The movement of water in plant cells takes place between the cellular vacuole and the extracellular environment.
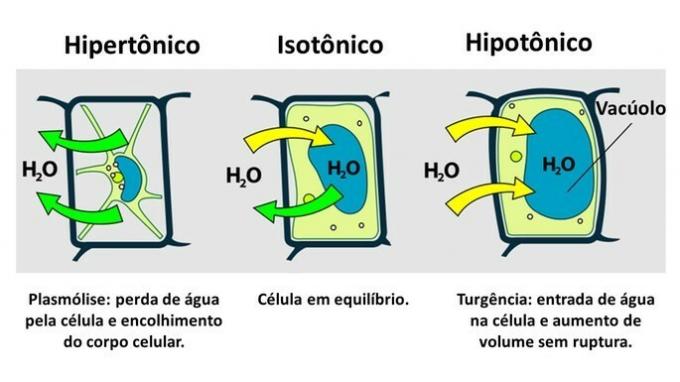
THE plant cell it has, in addition to the plasma membrane, a very resistant cell wall, which is formed by cellulose.
Therefore, unlike the animal cell, the plant cell resists disruption when it is inserted in a hypotonic environment, where water tends to enter the cell. The cell swells, increasing its volume, but the cell wall prevents rupture.
The loss of water by a plant cell, which is inserted in a hypertonic environment, is called plasmolysis. On the other hand, the entry of water into the vacuole when the cell is in a hypotonic medium is called turgency, when there is an increase in cell volume.
How does osmotic pressure influence osmosis?
A solute is any substance that can be diluted in a solvent, such as sugar dissolved in water. While osmotic pressure is the pressure made for water to move.
As osmosis is a process that occurs from the least concentrated (hypotonic) to the most concentrated (hypertonic) medium in search for balance, osmotic pressure is the pressure exerted on a system to prevent osmosis from happening naturally.
Therefore, the greater the concentration difference between the hypertonic and hypotonic media, the greater should be the osmotic pressure applied on the more concentrated solution to avoid osmosis.
Learn more about osmotic pressure.
What is reverse osmosis and how it works
Reverse osmosis is the passage of water in the opposite direction to osmosis. Thus, water moves from a more concentrated solution to a less concentrated one.
Reverse osmosis takes place by applying a pressure greater than the natural osmotic pressure.
As the semi-permeable membrane only allows the passage of solvent (pure water), it retains solutes.
An example of reverse osmosis is the transformation of salt water into fresh water by the desalination process.
Learn more about reverse osmosis.
Difference between osmosis and diffusion
Diffusion is the passage of very small molecules of gases and solutes dissolved in water through the plasma membrane. In this case, the solute molecules will shift from the more concentrated medium to the less concentrated medium. They move along a concentration gradient and spread out over the available space.
THE facilitated diffusion it is the passage, through the membrane, of substances that do not dissolve in lipids, with the help of proteins that permeate the lipid bilayer.
Like osmosis, diffusion is also considered a passive transport, as it occurs in favor of a concentration gradient.
Curiosity
The expression "learning by osmosis" is widely used by students who would like to learn new content without having to study, that is, without making an effort.
Read too:
- Solute and solvent
- Sodium and Potassium Pump
- Selective Permeability