In laboratories and chemical industries it is very important to know the amount of substances involved in the reactions. Therefore, below we will deal with the main concepts, quantities and measurement units related to the masses of atoms:
- Atomic Mass Unit (u):
The standard weight reference atom to designate the atomic mass unit is carbon-12 (12Ç). An atomic mass unit (1 u) corresponds to the value of 1.66054. 10-24 g, which is the mass of 1/12 of the carbon isotope with a mass equal to 12 (12Ç).
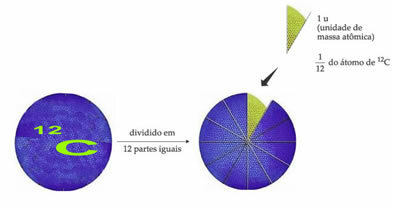
The atomic mass unit (u) is the unit used in all quantities listed below.
- Atomic Mass of an Atom or Isotope (MA):
It is obtained by comparing it with the reference value mentioned in the previous item. For example, the atomic mass of 919F is given by the mass spectrometer, which is equal to 18.9984 u. Rounding up, it equals 19, which is the same value as its mass number (A). This value means that the atomic mass of the element 919F is 19 times greater than the mass of 1/12 of the 12Ç.
- Atomic Mass of an Element (AM):
The atomic mass of the element is determined by the weighted average of the atomic masses of its isotopes. This is done by multiplying the atomic masses of each isotope by the percentage that appears in the element's constitution. These values are then added up, which are divided by 100, resulting in the total percentage.
Do not stop now... There's more after the advertising ;)
For example, the element neon is composed of three isotopes with the following atomic masses and percentages in their constitution:

Calculating the atomic mass (MA) of this element:
BADneon = (20. 90,92) + (21. 0,26) + (22. 8,82)
100
BADneon = 20,179 u
- Molecular Mass (MM):
As the name implies, it is used for molecular substances, that is, atoms linked through a sharing of pairs of electrons, which are called covalent bonds.
Molecular mass is obtained by multiplying the number of atoms of each element by their atomic masses and adding up the results.
For example, the CO molecule2 contains one carbon and two oxygens, so we'll multiply the atomic mass of carbon by 1; and oxygen by two, adding them later:
MMCO2 = (1. BADÇ) + (2. BADO)
MMCO2 = (1. 12) + (2. 16)
MMCO2 = 12 + 32
MMCO2 = 44 u
- Mass-Formula:
It is the same calculation performed for molecular mass, however, for ionic compounds. In this case the name is different, because they are not grouped into molecules, but into clusters of ions. As molecules do not exist, there is obviously no sense in talking about molecular mass, but the reasoning behind the calculation is the same.
Example:
NaCl
↓ ↓
23 +35.5 → Formula mass = 58.5 u
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Masses of atoms"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/massas-dos-atomos.htm. Accessed on June 28, 2021.