Scientist Friedrich Kohlrausch (1840-1910) was the first to propose that pure water conducts electricity, albeit on a small scale. This is because water behaves in an amphoteric way; that is, on certain occasions it acts like acid, donating protons (H+); and in others it behaves like a base, receiving protons.
This means that water performs its own ionization, according to the chemical equation shown below:
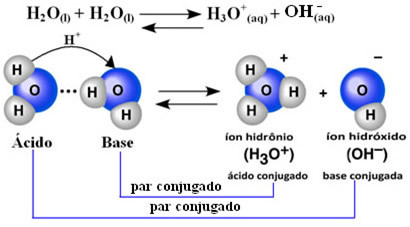
This process is called water autoionization and it occurs on a very small scale, that is, water is a very weak electrolyte, with low values of degree of ionization and ionization constant at equilibrium (Kç). This is exactly why water has such low conductivity.
To give you an idea, at an ambient temperature of 25°C it is possible to determine that the concentrations of hydroxide and hydronium ions produced in the self-ionization of pure water are equal to 1. 10-7 mol. L-1. This means that out of a billion water molecules, only two ionize.
The ionic balance constant of water is called çwater dissociation constant,
autoprotolysis constantor ionic product of water.This constant is represented by Kw, because the w refers to the word water, which in English means water.Do not stop now... There's more after the advertising ;)
Its calculation is done in the same way as the other equilibrium constants, remembering that, as stated in the text "Constants of Balance Kc and Kp”, in this case, only the products will appear in the expression, because water in the liquid state has the same activity to 1. Pure liquid or solid substances are not put into the expression of the dissociation constant because they do not change. Only aqueous and gaseous solutions are placed. So we have:
Kw = [H3O+]. [oh-]
Kw = (1. 10-7). (1. 10-7)
Kw = 10-14
As with the other equilibrium constants, OKw it only changes with the change in temperature. As the temperature of water increases, its ionization also increases, which means that the self-ionization of water is an endothermic process, that is, it absorbs heat.
This can be seen in the ionic product values of water (Kw) given in the table below at different temperatures:
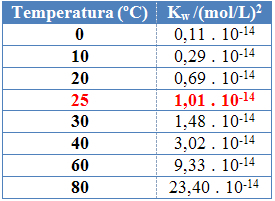
The ionic product of water will always have a fixed value at each temperature, whether in pure water or in solution. Even if the solution has concentrations of H ions3O+ and oh- different, the product between them will remain constant.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Ionic Water Product (Kw)"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/produto-ionico-Agua-kw.htm. Accessed on June 28, 2021.